No products in the cart.
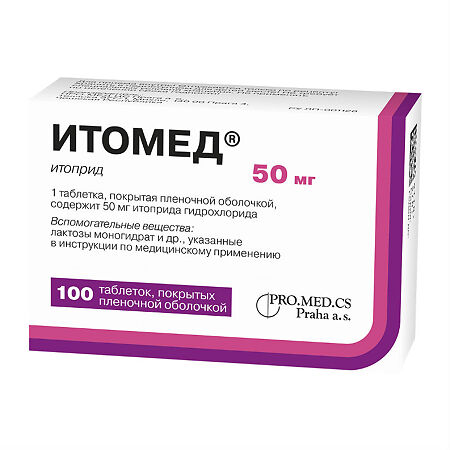
Itomed, 50 mg 100 pieces
€23.14 €19.29
EAN: 8595026435001
SKU: 224224
Categories: Medicine, Stomach, intestines, liver, Ulcer and gastritis
Description
Itomed – stimulates gastrointestinal tone and motility, antiemetic.
Pharmacodynamics
It enhances gastrointestinal motility due to antagonism with D2-dopamine receptors and inhibition of acetylcholinesterase. Activates the release of acetylcholine and inhibits its degradation.
It has antiemetic effect due to interaction with D2 receptors located in the trigger zone. Causes dose-dependent suppression of apomorphine-induced vomiting.
Activates propulsive gastric motility through antagonism with D2-receptors and dose-dependent inhibition of acetylcholinesterase activity.
It has a specific effect on the upper gastrointestinal tract, accelerates gastric transit and improves gastric emptying. It has no effect on serum concentrations of gastrin.
Pharmacokinetics
It is quickly and well absorbed in the gastrointestinal tract. The relative bioavailability of the drug is 60%.Cmax is 0.28 µg/mL, after administration of 50 mg of the drug Tmax in plasma is about 0.5-0.75 h. When repeatedly administered 50-200 mg 3 times daily for 7 days, pharmacokinetics is linear and cumulation is minimal.
It binds with plasma proteins (mainly with albumin) by 96%, with α1-acid glycoprotein by less than 15%. It is actively distributed in tissues (Vd – 6.1 l/kg) and found in high concentrations in the kidneys, small intestine, liver, adrenal glands, stomach. In therapeutic doses it slightly penetrates into brain and spinal cord, breast milk.
It is metabolized in the liver under the action of flavin-dependent monooxygenase. Three metabolites have been identified, one of which has negligible activity: 2-3% of the activity of ithopride.
Extracted by the kidneys. T1/2 is 6 h, in patients with trimethylaminuria T1/2 is increased.
Indications
Indications
Symptomatic treatment of functional non-ulcer dyspepsia (chronic gastritis):
flatulence;
gastralgia;
feeling of discomfort in the epigastric region;
anorexia;
heartburn;
nausea;
vomit.
Pharmacological effect
Pharmacological effect
Itomed – stimulating tone and motility of the gastrointestinal tract, antiemetic.
Pharmacodynamics
Strengthens gastrointestinal motility due to antagonism with D2-dopamine receptors and inhibition of acetylcholinesterase. Activates the release of acetylcholine and suppresses its destruction.
It has an antiemetic effect due to interaction with D2 receptors located in the trigger zone. Causes dose-dependent suppression of apomorphine-induced vomiting.
Activates propulsive gastric motility due to antagonism with D2 receptors and dose-dependent inhibition of acetylcholinesterase activity.
It has a specific effect on the upper gastrointestinal tract, accelerates gastric transit, and improves emptying. Has no effect on serum gastrin concentrations.
Pharmacokinetics
Quickly and well absorbed into the gastrointestinal tract. The relative bioavailability of the drug is 60%. Cmax is 0.28 mcg/ml, after taking 50 mg of the drug, Tmax in plasma is about 0.5–0.75 hours. With repeated administration of 50–200 mg 3 times a day for 7 days, the pharmacokinetics are linear, the accumulation is minimal.
Bound to plasma proteins (mainly albumin) by 96%, with α1-acid glycoprotein by less than 15%. Actively distributed in tissues (Vd – 6.1 l/kg) and found in high concentrations in the kidneys, small intestine, liver, adrenal glands, and stomach. In therapeutic doses, it slightly penetrates into the brain and spinal cord, and breast milk.
Metabolized in the liver under the action of flavin-dependent monooxygenase. 3 metabolites have been identified, one of which exhibits insignificant activity: 2–3% of the activity of itopride.
Excreted by the kidneys. T1/2 is 6 hours, in patients with trimethylaminuria T1/2 increases.
Special instructions
Special instructions
If symptoms of galactorrhea and gynecomastia occur, it is necessary to interrupt or completely stop treatment.
When treating primary biliary cirrhosis, transient decompensation of liver cirrhosis may occur, which disappears after discontinuation of the drug.
Impact on the ability to drive vehicles or perform work that requires increased speed of physical and mental reactions. During the treatment period, it is necessary to refrain from driving vehicles and engaging in potentially hazardous activities that require increased concentration and speed of psychomotor reactions.
Active ingredient
Active ingredient
Itopride
Composition
Composition
Active ingredient:
itopride hydrochloride 50 mg;
Excipients:
lactose monohydrate – 74.68 mg;
pregelatinized corn starch – 5 mg;
croscarmellose sodium – 1.8 mg;
colloidal silicon dioxide – 1 mg;
magnesium stearate – 2.52 mg;
Film shell:
Opadry 85F18422 white – 3 mg (polyvinyl alcohol, partially hydrolyzed – 1.2 mg, titanium dioxide – 0.75 mg, macrogol 4000 – 0.606 mg, talc – 0.444 mg)
Pregnancy
Pregnancy
Itopride is contraindicated during pregnancy.
When taking the drug in therapeutic doses, itopride slightly passes into breast milk, so you should stop taking the drug during breastfeeding (see “Contraindications”).
Contraindications
Contraindications
hypersensitivity to itopride or any auxiliary component of the drug;
gastrointestinal bleeding, mechanical obstruction and perforation of the gastrointestinal tract;
lactase deficiency, lactose intolerance, glucose-galactose malabsorption;
pregnancy;
lactation period;
children’s age (up to 16 years);
With caution: patients taking cholinesterase inhibitors and m-cholinomimetics, as well as elderly patients with reduced liver and kidney function.
Side Effects
Side Effects
From the hematopoietic organs: leukopenia, thrombocytopenia.
From the endocrine system: gynecomastia, hyperprolactinemia.
From the digestive system: increased salivation, nausea, diarrhea, constipation, jaundice, pain in the epigastric region.
From the nervous system: headache, sleep disturbance, irritability, dizziness, tremor.
Allergic reactions: urticaria, anaphylactic shock.
Laboratory indicators: increased activity of AST, ALT, GGT, alkaline phosphatase, hyperbilirubinemia.
Interaction
Interaction
Accelerates the absorption of other drugs.
The prokinetic effect of the drug does not change under the influence of agents that reduce the acidity of gastric juice (cimetidine, ranitidine, teprenone, cetraxate).
M-anticholinergic agents reduce the effectiveness of itopride.
The cholinergic effects of itopride may be increased by concomitant use of m-cholinomimetics and cholinesterase inhibitors.
Overdose
Overdose
Treatment: gastric lavage and symptomatic therapy.
Storage conditions
Storage conditions
In a dry place, protected from light, at a temperature of 15–25 °C.
Shelf life
Shelf life
3 years
Manufacturer
Manufacturer
PRO.MED.CS Prague, Czech Republic
Additional information
| Shelf life | 3 years |
|---|---|
| Conditions of storage | In a dry, light-protected place at 15-25 °C. |
| Manufacturer | PRO.MED.CS Prague, Czech Republic |
| Medication form | pills |
| Brand | PRO.MED.CS Prague |
Other forms…
Related products
Buy Itomed, 50 mg 100 pieces with delivery to USA, UK, Europe and over 120 other countries.