No products in the cart.
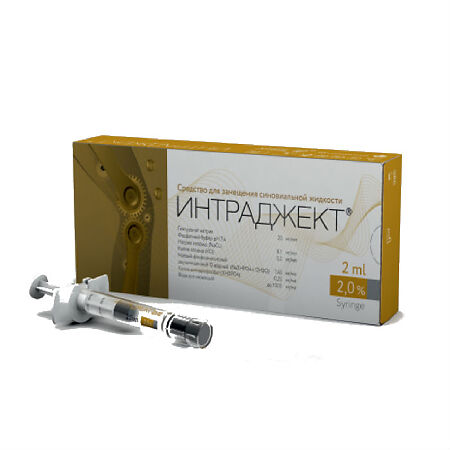
Intraject 2% 2 ml
€1.00
Out of stock
(E-mail when Stock is available)
Description
Hyaluronic acid is a natural polysaccharide belonging to the class of glycosaminoglycans, which is part of all body tissues and is the most important structural element of synovial fluid. Hyaluronic acid is unmodified.
The product INTRAJECT® contains
Hyaluronic acid sodium salt and water, which molecules form hydrogen bonds due to the presence of
./in the structure of the molecules of hydroxyl groups (OH-groups), as well as due to the presence of a large number of nitrogen atoms in the hyaluronic acid molecule. Due to the formation of hydrogen bonds with water molecules, hyaluronic acid has a high ability to bind and retain water, acquiring a jelly-like structure.
Macromolecules of hyaluronic acid, thanks to the same mechanisms, form intramolecular and intermolecular interactions, giving a fairly stable three-dimensional structure.
The INTRAGEKT® product has an ordered gel structure that is able to retain hyaluronic acid molecules and water inside the synovial sac and provides stability of the INTRAGEKT® product, which guarantees the prolonged action of the product.
The viscoelastic properties of hyaluronic acid in synovial fluid provide lubrication and cushioning of articular surfaces. Synovial fluid in joints with degenerative diseases (osteoarthritis) or traumatic joint changes, has lower viscosity and elasticity than in healthy joints. Hyaluronic acid, a component of INTRAGEKT®, has a molecular weight of 2.2 million daltons, close to natural human hyaluronic acid, and has a positive effect after injection in the diagnosis of osteoarthritis (osteoarthritis). Intra-articular injection of INTRAJECT® restores physiological and rheological properties of synovial fluid of the joint affected by osteoarthritis. As a consequence, it reduces pain and discomfort and improves mobility in the joint.
Indications
Indications
INTRAGECT® is intended for intra-articular administration only.
The INTRAGENT® product is used:
1. For temporary replacement and replenishment of synovial fluid.
2. for relief of pain and stiffness caused by degenerative-dystrophic (osteoarthritis or osteoarthritis) and traumatic changes in knee, hip and other synovial joints.
3. for use in patients with an active lifestyle and who regularly load the affected joint.
Active ingredient
Active ingredient
Composition
Composition
1. Sodium hyaluronate
2. Phosphate buffer pH 7.4
Composition of dry matter, mg/ml (%):
– Sodium chloride – 8.1 mg (81.0%) NaCL
– Potassium chloride – 0.2 mg (2.00%) KCl
– Sodium phosphate divalent 12 aqueous 1.45 mg (14.50 %) Na2HPO4 x 12H2O
– Potassium dihydrophosphate – 0.25 mg (2.50 %) KH2PO4
How to take, the dosage
How to take, the dosage
Recommended course of administration of INTRAJECT® in patients with moderate to moderate osteoarthritis:
In order to gradually fill the synovial space of the joint, INTRAJECT® is administered in a course of 3 to 5 injections with one week intervals between each injection.
The number of injections and the duration of the course of administration is determined by the physician; the recommended maximum duration of administration is 6 weeks.
The period between courses of injections of INTRAJECT® is determined by the physician and a break of 6 to 12 months is recommended.
The simultaneous use of INTRAGENT® for multiple joints is possible.
The use of INTRAGEKT® has an effect only on the affected joint where it is injected.
Interaction
Interaction
The efficacy and safety of INTRAJECT® in combination with simultaneous intra-
Joint administration of other medical devices and/or medications has not been studied, therefore the combined administration of INTRAJECT® with other medical devices and/or medications is possible only if the safety and efficacy of this method has been proven.
Incompatibilities have been noted between sodium hyaluronate and quaternary ammonium salts such as benzalkonium chloride. Therefore, under no circumstances should INTRAJECT® come in contact with these products or with medical and surgical instruments that have been treated with these antiseptics.
Special Instructions
Special Instructions
Consultation with a physician is required before using a medical product.
The instructions for use should be read carefully before using INTRAJECT®.
In preparation for and during use of the medical device, strict adherence to asepsis is required as there may be a risk of infection.
The intravascular administration of INTRAJECT® is not permitted.
Ingestion of INTRAJECT® should be avoided in the joint capsule, surrounding tissues or blood vessels.
For 48 to 72 hours after the application of INTRAJECT® to the joint, do not perform physiotherapy, taping, pressure dressing with medication, prolonged static stress on the limb, do not assume a deep squat posture and avoid running, jumping or carrying heavy objects.
Synopsis
Synopsis
The INTRAGEKT® product is a sterile, colorless, transparent, homogeneous, viscous gel without mechanical impurities obtained by bacterial fermentation.
The INTRAGEKT® product does not contain any animal proteins and does not require a prior allergy test.
Hyaluronic acid is a natural glycosaminoglycan of the intercellular matrix which has the greatest hygroscopic properties of all known natural mucopolysaccharides.
Contraindications
Contraindications
1. Hypersensitivity or allergy to components of INTRAJECT®.
2. History of autoimmune disease or autoimmune therapy.
3. Pathological bleeding (endogenous or caused by the use of anticoagulants).
4. Infectious (septic) inflammatory process in the joint or periarticular tissues, intraarticular effusion, general infectious disease.
5. Presence of evidence of active skin disease or skin infection in the immediate vicinity of the
injection site.
6. Pregnancy and lactation.
7. children under 18 years of age.
Side effects
Side effects
The intra-articular administration of INTRAJECT® is well tolerated. In rare cases there may be local secondary effects: pain, sensation of heat, redness, swelling of the joint, the appearance of intra-articular exudation, very rarely allergic reactions. Side effects may occur immediately or after some time. These phenomena are transient and go away spontaneously. If these symptoms persist for more than one week, the patient should see a physician. In exceptional cases septic arthritis may occur, also unrelated to application of INTRAJECT®, symptoms of this complication are: local inflammatory reaction, increased joint pain, increased body temperature.
Pregnancy use
Pregnancy use
There are no data on the safety of INTRAJECT® in pregnancy and lactation. Use of INTRAJECT® in pregnancy and lactation is with caution and at the discretion of the physician.
Additional information
| Shelf life | 3 years from date of manufacture. |
|---|---|
| Conditions of storage | Do not use the product with damaged packaging. Do not use after the expiration date. Keep out of the reach of children. |
| Manufacturer | FBK LLC, Russia |
| Medication form | solution for injection |
| Brand | FBK LLC |
Related products
Buy Intraject 2% 2 ml with delivery to USA, UK, Europe and over 120 other countries.