No products in the cart.
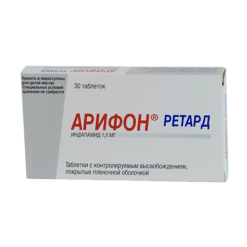
Indapamide-CRCa, 1.5 mg 60 pcs
€8.17 €6.81
Out of stock
(E-mail when Stock is available)
Description
Tiazide-like diuretic, antihypertensive agent.
It decreases tone of arterial smooth muscle, decreases PPS, also has moderate saluretic activity due to disruption of reabsorption of sodium, chlorine and water ions in the cortical segment of the Genle loop and proximal convoluted tubule of nephron.
The decrease of RPS is due to several mechanisms: reduction of the sensitivity of the vascular wall to noradrenaline and angiotensin II; increased synthesis of prostaglandins with vasodilatory activity; inhibition of calcium ions influx into the smooth muscle elements of the vascular wall. In therapeutic doses has almost no effect on lipid and carbohydrate metabolism.
Hypotensive effect appears only at initially elevated BP, develops by the end of the first week and reaches a maximum after 3 months of systematic use.
Indications
Indications
Active ingredient
Active ingredient
Composition
Composition
How to take, the dosage
How to take, the dosage
Take orally 2.5 mg once daily (in the morning). If hypotensive effect is not sufficiently pronounced after 2 weeks of treatment, the dose is increased to 5-7.5 mg/day.
The maximum daily dose is 10 mg divided into 2 doses (in the morning).
Interaction
Interaction
Concomitant use of GCS, tetracosactide for systemic use decreases the hypotensive effect due to water and sodium ion retention under the influence of GCS.
Concomitant use with ACE inhibitors increases the risk of hyponatremia.
Concomitant use with NSAIDs (for systemic use) may reduce the hypotensive effect of indapamide. If significant fluid loss occurs, acute renal failure may develop (due to a sharp decrease in glomerular filtration).
In concomitant use with calcium preparations hypercalcemia may occur due to decreased excretion of calcium ions in the urine.
Concomitant use with cardiac glycosides, corticosteroids increases the risk of hypokalemia.
Concomitant use of agents that may cause hypokalemia (amphotericin B, gluco- and mineralocorticoids, tetracosactide, laxatives that stimulate intestinal peristalsis) increases the risk of hypokalemia.
Concomitant use with tricyclic antidepressants (including imipramine) increases the hypotensive effect and increases the risk of orthostatic hypotension (additive effect).
Concomitant use with astemizole, napridil, erythromycin (IV), pentamidine, sultopride, terfenadine, vincamine, quinidine, disopyramide, amiodarone, brettilia tozilate, sotalol increases the risk of pirouette arrhythmia.
Concomitant use with baclofen increases the hypotensive effect.
Concomitant use with halofantrine increases the risk of cardiac arrhythmias (including pirouette-type ventricular arrhythmias).
Simultaneous use with lithium carbonate increases the risk of lithium toxicity with decreased renal clearance.
Concomitant use with metformin may lead to lactic acidosis, which appears to be associated with the development of functional renal failure due to the action of diuretics (mainly loop diuretics).
Concomitant use with cyclosporine may increase plasma creatinine, which is observed even when water and sodium ions are normal.
Special Instructions
Special Instructions
With caution, use in patients with diabetes mellitus (glucose control is necessary, especially in the presence of hypokalemia), gout (possible increase in the number of attacks), in patients with a history of allergic reactions to sulfonamide derivatives.
The electrolyte level in plasma (potassium, sodium, calcium) should be controlled during treatment.
Contraindications
Contraindications
Side effects
Side effects
Digestive system disorders: nausea, feeling of discomfort or pain in the epigastrium.
CNS disorders: weakness, fatigue, dizziness, nervousness.
Cardiovascular system: orthostatic hypotension.
Metabolism: hypokalemia, hyperuricemia, hyperglycemia, hyponatremia, hypochloremia.
Allergic reactions: skin manifestations.
Similarities
Similarities
Additional information
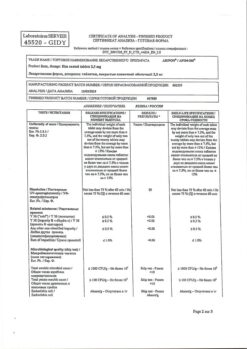
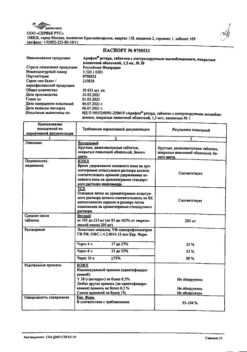
| Manufacturer | KRKA-RUS, Russia |
|---|---|
| Medication form | sustained release tablets |
| Brand | KRKA-RUS |
Other forms…
Related products
Buy Indapamide-CRCa, 1.5 mg 60 pcs with delivery to USA, UK, Europe and over 120 other countries.