No products in the cart.
Imuron-vac, lyophilizate 8-15 ml/mg 50 mg 2 pcs
€1.00
Out of stock
(E-mail when Stock is available)
Description
Immuron-vac represents a culture of microbial cells of BCG-1 vaccine strain (M.bovis BCG-1, Russia) lyophilized in 1.5% sodium glutamate monohydrate solution.
Immuron-vac stimulates the immune system and has antitumor activity. During intravesical instillation BCG acts as a nonspecific immunomodulator, causing: a whole complex of immune reactions, which involves a number of immune system cells, including T and B lymphocytes, macrophages, a number of cytokines.
Indications
Indications
Treatment of non-invasive urothelial bladder cancer:
– treatment of pre-invasive cancer stage Tis (carcinoma in situ).
Prevention of recurrence of bladder cancer after radical treatment:
– stage Ta (carcinoma infiltrating only the mucous membrane of the bladder): Ta G1–G2 with a multifocal and/or recurrent tumor; Ta G3;
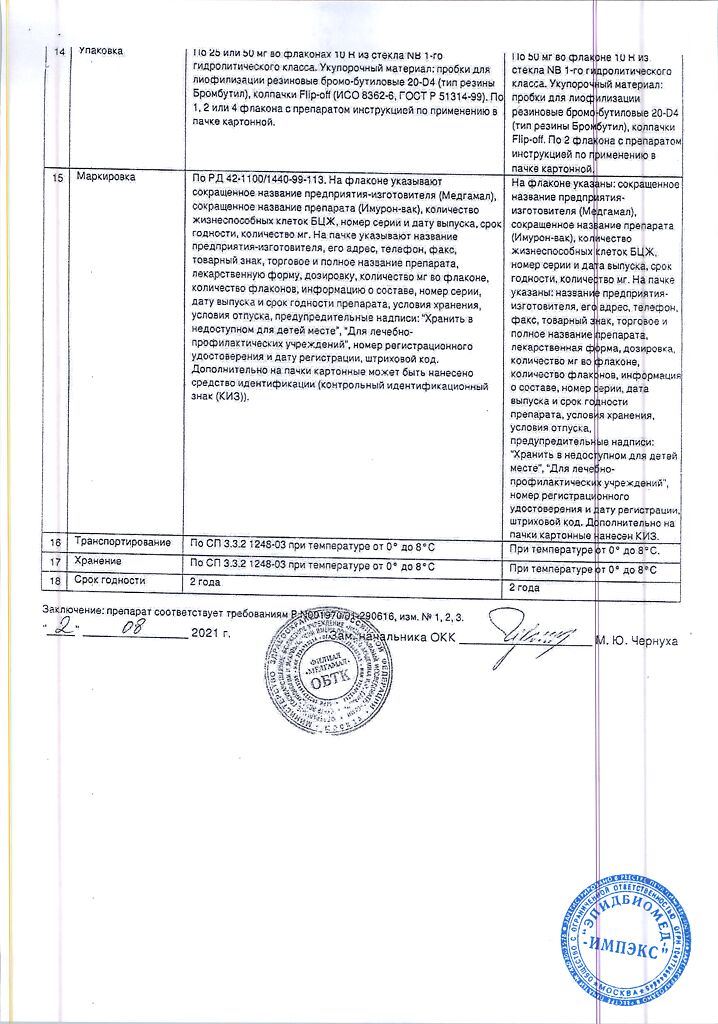
– stage T1 (tumor infiltrating the subepithelial connective tissue and not affecting the muscular layer of the bladder);
– stage Tis (preinvasive carcinoma, carcinoma in situ).
Pharmacological effect
Pharmacological effect
Imuron-vac is a culture of microbial cells of the vaccine strain BCG-1 (M. bovis BCG -1, Russia), lyophilized in a 1.5% solution of sodium glutamate monohydrate.
Imuron-vac stimulates the immune system and has antitumor activity. When administered intravesically, BCG acts as a nonspecific immunomodulator, causing: a whole complex of immune reactions, which involve a number of cells of the immune system, including T and B lymphocytes, macrophages, and a number of cytokines.
Special instructions
Special instructions
The BCG vaccine should not be used for subcutaneous, intravenous, intramuscular or intravenous administration, or for vaccination purposes.
The patient should be warned about the possibility of complications of BCG therapy. If systemic reactions to the BCG vaccine occur, consultation with a phthisiatrician is necessary.
Adverse reactions are common with BCG vaccine for immunotherapy of bladder cancer, but these reactions are usually not severe and are usually transient. With an increase in the number of instillations, the risk of side effects increases.
When prescribing BCG therapy, it is necessary to take into account the risk of developing severe systemic BCG reactions/infections, which include: fever >39.5 °C for at least 12 hours; fever with temperature >38.5°C for at least 48 hours; miliary pneumonia caused by BCG; granulomatous hepatitis; deviations in indicators characterizing liver function; organ dysfunction (not related to the genitourinary system) with granulomatous inflammation confirmed by biopsy; Reiter’s syndrome.
Traumatic instillation can lead to BCG-related sepsis with possible development of septic shock and risk of death.
Before each intravesical instillation of the BCG vaccine, a urinary tract infection should be excluded (inflammation of the bladder mucosa may increase the risk of hematogenous spread of BCG infections). If a urinary tract infection is detected during BCG vaccine therapy, treatment should be interrupted until the course of antibiotic therapy is completed and the urine test is normalized.
Infections of implants and grafts have been reported in patients, such as those with an aneurysm.
Isolated cases have been reported in which BCG bacteria persisted in the urinary tract for more than 16 months.
In the case of fever or gross hematuria, administration of the vaccine should be delayed until these symptoms have resolved.
Patients with reduced bladder capacity have an increased risk of bladder shrinkage.
HLA-B27 (human leukocyte antigen B27) positive patients have an increased risk of developing reactive arthritis or Reiter’s syndrome.
The drug should not be used in the same premises, as well as by those employees who are associated with the preparation of cytotoxic drugs for parenteral administration. Persons with established immunodeficiency should not work with the drug. Contact of the drug with the skin and mucous membranes should be avoided.
Intravesical therapy with the drug may cause the development of sensitivity to tuberculin and subsequently complicate the interpretation of tuberculin skin tests in the diagnosis of mycobacterial infection. Therefore, a tuberculin test should be performed before administering the vaccine.
Sexual transmission. Cases of sexual transmission of BCG infection have not yet been recorded, however, a condom should be used during sexual intercourse for a week after use.
Patients undergoing treatment are advised to thoroughly wash their hands and genitals after urinating.
A spilled solution of the drug must be neutralized using the methods and regimes specified in the current sanitary standards and rules for the disinfection of mycobacteria.
In case of skin contact, the contaminated area must be treated with a suitable disinfectant.
Active ingredient
Active ingredient
Vaccine for the treatment of bladder cancer BCG
Composition
Composition
Active substance:
Contraindications
Contraindications
Increased sensitivity; active form of tuberculosis; previous tuberculosis; the size of the local reaction to the intravenous injection of tuberculin at a dose of 2 TU (Mantoux test) is 17 mm or more; congenital or acquired immunodeficiency as a result of concomitant diseases (for example, HIV infection, leukemia, lymphoma), treatment of malignant neoplasms (for example, cytostatics, radiation) or immunosuppressive therapy (for example, corticosteroids); acute cystitis or gross hematuria (until the clinical manifestations disappear); bladder perforation; severe concomitant diseases in the stage of decompensation.
Traumatic catheterization or the appearance of blood after bladder catheterization are contraindications for BCG instillation on a given day.
BCG therapy can be started no earlier than 2-3 weeks after transurethral resection of the bladder (TURP), bladder biopsy or traumatic catheterization (depending on the speed of wound healing).
Not recommended for use in children due to lack of data on efficacy and safety.
Side Effects
Side Effects
Side effects are grouped into groups based on frequency of occurrence, and within each group the effects are arranged in decreasing order of severity.
Very common (>1/10)
Common complications: discomfort, nausea, malaise, chills, fever.
Urinary tract diseases: cystitis and inflammatory (granulomatous) reactions in the bladder, frequent urination, accompanied by discomfort and pain.
Reproductive system disorders: asymptomatic granulomatous prostatitis.
From the immune system: transient systemic reaction to BCG (fever with temperature up to 38.5 °C, flu-like symptoms).
Often (>1/100, <1/10)
General disorders: fever with a temperature above 38.5 °C.
Uncommon (>1/1000, <1/100)
Infections: development of severe systemic BCG reactions/infections.
From the hematopoietic system: cytopenia, most often erythropenia, manifested as anemia.
From the immune system: Reiter’s syndrome (conjunctivitis, asymmetric oligoarthritis, urethritis).
Respiratory system disorders: miliary pneumonia, pulmonary granuloma.
From the hepatobiliary system: hepatitis.
Skin changes: skin rash, skin abscess.
From the musculoskeletal system: arthritis, arthralgia, in most cases a manifestation of a hypersensitivity reaction to BCG.
From the urinary system: development of urinary tract infections, gross hematuria, bladder retraction, urinary tract obstruction, bladder contracture.
From the genitourinary system: orchitis, epididymitis, granulomatous prostatitis with severe symptoms.
General disorders: decreased blood pressure.
Rarely (>1/10000, <1/1000)
Vascular disorders: development of infectious vascular lesions (for example, infected aneurysm).
Kidney diseases: kidney abscess.
Infections: BCG-associated sepsis.
Very rare (< 1/10000), including isolated reports
Infections: BCG infection of implants and adjacent tissues (for example, infection of an aortic graft, cardiac defibrillator, hip or knee arthroplasty).
From the lymphatic system: cervical lymphadenitis, infection of the pelvic lymph nodes.
From the immune system: allergic reactions (manifested in the form of symptoms such as swelling of the eyelids).
From the organs of vision: chorioretinitis, conjunctivitis, uveitis.
Vascular disorders: vascular fistula.
Gastrointestinal disorders: vomiting, intestinal fistula, peritonitis.
Damage to the musculoskeletal system and connective tissue: bone marrow infection, osteomyelitis, lumbar muscle abscess.
From the genitourinary system: orchitis, epididymitis, resistant to anti-tuberculosis therapy, development of infection of the head of the penis.
Frequency unknown (cannot be estimated from available data)
Reproductive system disorders: symptoms may develop, such as vaginal pain, dyspareunia (pain during sexual intercourse).
Adverse reactions during therapy with the BCG vaccine are observed frequently, but are usually not severe and are usually transient. With an increase in the number of instillations, the risk of side effects increases.
Arthritis/arthralgia and skin rash that sometimes develop may be manifestations of hypersensitivity reactions to BCG. In such cases, discontinuation of drug therapy may be necessary.
Local adverse reactions
Unpleasant sensations and pain when urinating, as well as frequent urination, are observed in 90% of patients. Cystitis and an inflammatory (granulomatous) reaction may be an integral part of the antitumor activity of the drug.
Transient systemic BCG reactions
After instillation of the BCG vaccine, there may be an increase in body temperature up to 38.5 ° C, flu-like symptoms and general malaise – these symptoms usually subside after 24-48 hours; in case of their development, standard symptomatic treatment is necessary. These symptoms are signs of an incipient immune reaction. All patients prescribed instillations should be closely monitored and instructed to report all episodes of fever and other non-genitourinary reactions.
Severe systemic adverse reactions/infections
Systemic reactions/infections include fever with a temperature greater than 39.5°C for at least 12 hours; fever with a temperature above 38.5 °C for at least 48 hours; miliary pneumonia caused by BCG; granulomatous hepatitis; deviations in indicators characterizing liver function; dysfunction of internal organs other than the genitourinary system caused by granulomatous inflammation confirmed by biopsy; Reiter’s syndrome. Severe infections caused by the BCG vaccine can lead to sepsis.
Therapeutic recommendations for the treatment of symptoms and syndromes that occur during therapy with the BCG vaccine
1) Symptoms of bladder irritation lasting no more than 48 hours. Symptomatic treatment is carried out.
2) Symptoms of bladder irritation lasting ≥48 hours. Vaccine therapy should be interrupted and treatment with a quinolone should be initiated. If after 10 days complete disappearance of symptoms is not observed, then it is necessary to prescribe a course of treatment with isoniazid* for a duration of 3 months.
In case of anti-tuberculosis therapy, vaccine treatment should be discontinued.
3) Concomitant bacterial urinary tract infection. Treatment with the vaccine should be postponed until the course of antibacterial therapy is completed and urine analysis is normalized.
4) Other undesirable effects from the genitourinary tract: granulomatous prostatitis, epididymitis and orchitis, urinary tract obstruction, kidney abscess. Cancel vaccine treatment.
Prescribe a course of treatment with isoniazid* and rifampicin* for 3–6 months, depending on the severity of the case.
In case of anti-tuberculosis therapy, vaccine treatment should be discontinued.
5) Fever with temperature < 38.5 °C for less than 48 hours. Symptomatic treatment with paracetamol.
6) Skin rash, arthralgia or arthritis, Reiter’s syndrome. Interrupt vaccine therapy.
Prescribe antihistamines or NSAIDs.
If there is no response to therapy, prescribe a course of treatment with isoniazid* for 3 months.
In case of anti-tuberculosis therapy, vaccine treatment should be permanently discontinued.
7) Systemic BCG reaction/infection without signs of septic shock. Cancel vaccine therapy.
Consider consulting with an infectious disease specialist. Prescribe a 6-month course of anti-tuberculosis therapy* using three drugs.
8) Systemic BCG reaction/infection with signs of septic shock. Cancel vaccine treatment.
Immediately prescribe a course of anti-tuberculosis therapy* with three drugs in combination with high-dose fast-acting corticosteroids.
Consult an infectious disease specialist.
* Caution: BCG bacteria are sensitive to all modern anti-tuberculosis drugs, except pyrazinamide. If three-drug anti-tuberculosis therapy is required, a combination of isoniazid, rifampicin and ethambutol is usually recommended.
Interaction
Interaction
During a course of intravesical instillations of Imuron-vac, the simultaneous administration of drugs to which mycobacteria BCG are sensitive should be avoided: anti-tuberculosis drugs, fluoroquinolones, doxycycline or gentamicin.
Overdose
Overdose
There is no evidence that an overdose can cause symptoms other than the adverse reactions described.
Storage conditions
Storage conditions
In a place protected from light, at a temperature of 0–8 °C. In accordance with SP 3.3.2.1248-03
Manufacturer
Manufacturer
Research Institute of EM named after Gamaleya, Russia
Additional information
| Conditions of storage | In a light-protected place at 0-8 °C. According to SP 3.3.2.1248-03 |
|---|---|
| Manufacturer | Gamaleya Research Institute of EM, Russia |
| Medication form | lyophilizate |
| Brand | Gamaleya Research Institute of EM |
Related products
Buy Imuron-vac, lyophilizate 8-15 ml/mg 50 mg 2 pcs with delivery to USA, UK, Europe and over 120 other countries.