No products in the cart.
Hyrabezol, 20 mg 30 pcs.
€23.40 €19.50
EAN: 8904102204347
SKU: 294644
Categories: Medicine, Stomach, intestines, liver, Ulcer and gastritis
Description
A gastric gland secretion reducing agent – proton pump inhibitor.
Pharmacodynamics
. An antiulcer agent from the group of proton pump inhibitors (H+/K+-ATPase), metabolized in the parietal cells of the stomach to active sulfonamide derivatives, which inactivate the sulfhydryl groups of H+/K+-ATPase.
Blocks the final stage of hydrochloric acid secretion, reducing basal and stimulated secretion, regardless of the nature of the stimulus.
It has high lipophilicity, easily penetrates into the parietal cells of the stomach and concentrates in them with cytoprotective effect.
The antisecretory effect after oral administration of 20 mg occurs within 1 hour and reaches its maximum after 2-4 hours; inhibition of basal and food stimulated acid secretion after 23 hours after the first dose is 62% and 82%, respectively; duration of action is 48 hours. After the end of the intake, secretory activity normalizes within 2-3 days.
In the first 2-8 weeks of therapy, serum gastrin concentration increases and returns to baseline levels within 1-2 weeks after discontinuation of the drug. It does not affect the central nervous system, cardiovascular and respiratory systems.
Pharmacokinetics
Absorption occurs in the small intestine (due to the presence of acid-resistant enteric coating) is high, time to reach maximum concentration – 3.5 hours. Values of maximum concentration (Cmax) and area under the curve “concentration of active substance – time” (AUC) are linear in the dose range from 10 to 40 mg. Metabolized in the liver with the participation of cytochrome P-450 CYP2C19 and CYP3A4 isoenzymes. Bioavailability is 52% and does not increase with repeated administration. Period of semi-elimination (T1/2) – 0.7-1.5 hour, clearance – 283 ± 98 ml/min.
In patients with chronic hepatic insufficiency of mild or moderate degree after a single use AUC is increased by 2 times, T1/2 – 2-3 times. After rabeprazole administration during 7 days the AUC is increased 1.5 times, T1/2 – 1.2 times.
In patients with stable terminal renal failure requiring hemodialysis (creatinine clearance less than 5 ml/min/1.73 m2) the distribution of rabeprazole sodium is close to that of healthy persons.
In elderly patients after rabeprazole administration for 7 days the AUC is 2 times higher, Cmax is 60% higher than in young patients.
Binding to plasma proteins is 97%.
Excreted by the kidneys – 90% as two metabolites: mercapturic acid conjugate (M5) and carbolic acid (M6); by the intestine – 10%. In patients with delayed CYP2C19 metabolism after 7 days of taking rabeprazole at the dose of 20 mg per day AUC is increased 1.9 times and half-life 1.6 times as compared with the same parameters in “fast metabolizers”, while Cmax is increased by 40%.
Indications
Indications
Gastric ulcer in the acute stage and anastomotic ulcer;
Pharmacological effect
Pharmacological effect
A drug that reduces the secretion of gastric glands – a proton pump inhibitor.
Pharmacodynamics
An antiulcer agent from the group of proton pump inhibitors (H+/K+-ATPase), is metabolized in the parietal cells of the stomach to active sulfonamide derivatives, which inactivate the sulfhydryl groups of H+/K+-ATPase.
Blocks the final stage of hydrochloric acid secretion, reducing the content of basal and stimulated secretion, regardless of the nature of the stimulus.
It is highly lipophilic, easily penetrates into the parietal cells of the stomach and concentrates in them, exerting a cytoprotective effect.
The antisecretory effect after oral administration of 20 mg occurs within 1 hour and reaches a maximum after 2-4 hours; inhibition of basal and food-stimulated acid secretion 23 hours after taking the first dose is 62% and 82%, respectively; duration of action – 48 hours. After the end of administration, secretory activity normalizes within 2-3 days.
In the first 2-8 weeks of therapy, the concentration of gastrin in the blood serum increases and returns to initial levels within 1-2 weeks after discontinuation of the drug. Does not affect the central nervous system, cardiovascular and respiratory systems.
Pharmacokinetics
Absorption occurs in the small intestine (due to the presence of an acid-resistant enteric coating) is high, the time to reach the maximum concentration is 3.5 hours. The values of the maximum concentration (Cmax) and the area under the active substance concentration-time curve (AUC) are linear in the dose range from 10 to 40 mg. Metabolized in the liver with the participation of cytochrome P-450 isoenzymes CYP2C19 and CYP3A4. Bioavailability – 52%, does not increase with repeated doses. Half-life (T1/2) – 0.7-1.5 hours, clearance – 283 ± 98 ml/min.
In patients with mild or moderate chronic liver failure, after a single dose, AUC increases by 2 times, T1/2 by 2-3 times. After taking 20 mg of rabeprazole for 7 days, AUC increases by 1.5 times, T1/2 – by 1.2 times.
In patients with stable end-stage renal disease requiring hemodialysis (creatinine clearance less than 5 ml/min/1.73 m2), the distribution of rabeprazole sodium is close to that in healthy individuals.
In elderly patients, after taking rabeprazole for 7 days, AUC is 2 times greater, Cmax is 60% greater than in young patients.
Connection with plasma proteins – 97%.
Excreted by the kidneys – 90% in the form of two metabolites: a conjugate of mercapturic acid (M5) and carbolic acid (M6); intestines – 10%. In patients with slow metabolism of CYP2C19, after 7 days of taking rabeprazole at a dose of 20 mg per day, the AUC increases by 1.9 times and the half-life by 1.6 times compared with the same parameters in “rapid metabolizers,” while Cmax increases by 40%.
Special instructions
Special instructions
The patient’s response to rabeprazole therapy does not exclude the presence of malignant neoplasms in the stomach.
Active ingredient
Active ingredient
Rabeprazole
Composition
Composition
Active ingredient:
Pregnancy
Pregnancy
Rabeprazole should not be prescribed to pregnant women (there are no data on the safety of rabeprazole during pregnancy). Breastfeeding should be stopped during treatment.
Contraindications
Contraindications
Hypersensitivity to rabeprazole, substituted benzimidazoles or other auxiliary components of the drug; pregnancy; lactation period; children’s age (up to 12 years).
Side Effects
Side Effects
Based on the experience of clinical trials, it can be concluded that rabeprazole is generally well tolerated by patients. Side effects are generally mild or moderate and are transient.
Interaction
Interaction
Rabeprazole slows down the excretion of certain drugs metabolized in the liver by microsomal oxidation (diazepam, phenytoin, indirect anticoagulants).
Overdose
Overdose
Symptoms:
Data on intentional or accidental overdose are minimal. There have been no cases of severe overdose of rabeprazole.
Recommendations for use
Recommendations for use
Khairabezol tablets should not be chewed or crushed. The tablets should be swallowed whole. It has been established that neither time of day nor food intake affects the activity of rabeprazole.
Storage conditions
Storage conditions
In a dry place, protected from light, at a temperature from 8 ° C to 25 ° C.
Shelf life
Shelf life
3 years. Do not use after the expiration date stated on the package.
Manufacturer
Manufacturer
Highglans Laboratories Pvt. Ltd, India
Additional information
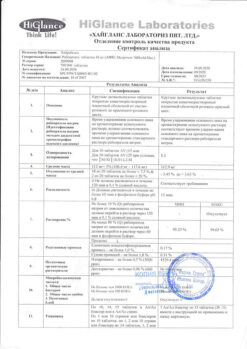
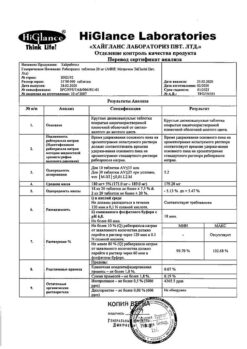
| Shelf life | 3 years. Do not use after the expiration date printed on the package. |
|---|---|
| Conditions of storage | In a dry, light-protected place at a temperature of 8 ° C to 25 ° C. |
| Manufacturer | Hyglans Laboratories Pvt. Ltd, India |
| Medication form | enteric-soluble film-coated tablets |
| Brand | Hyglans Laboratories Pvt. Ltd |
Other forms…
Related products
Buy Hyrabezol, 20 mg 30 pcs. with delivery to USA, UK, Europe and over 120 other countries.