No products in the cart.
Guttalax, tablets 5 mg 20 pcs
€11.03 €9.65
Description
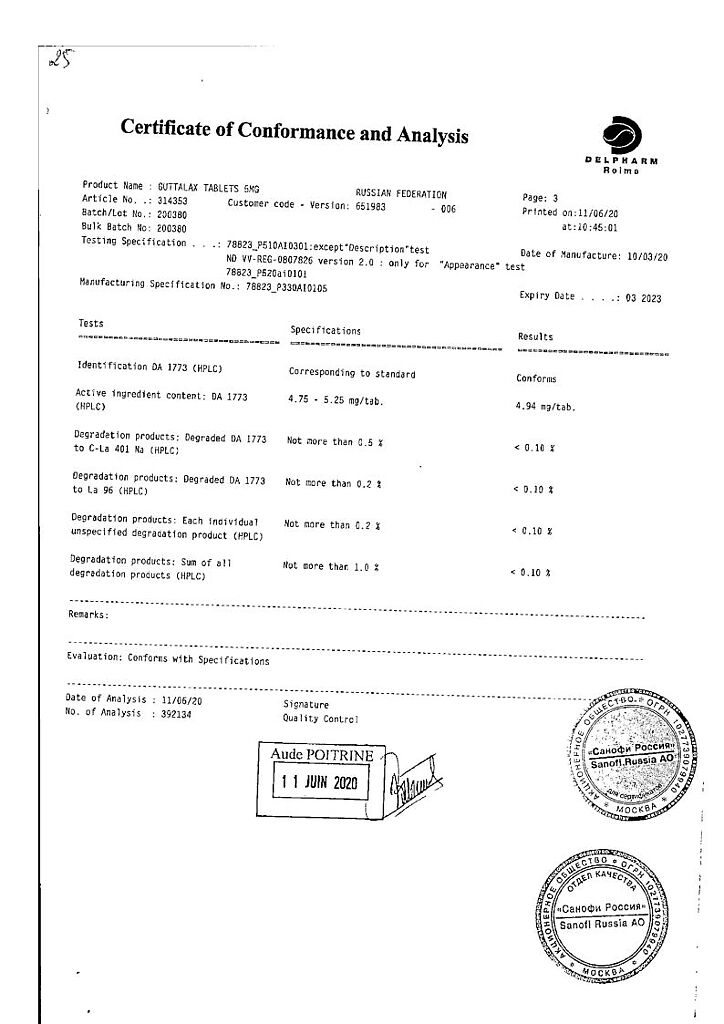
Laxative drug. The active ingredient – sodium picosulfate – is a laxative of triarylmethane group.
As a local laxative sodium picosulfate after bacterial cleavage in the large intestine has a stimulating effect on the mucosa of the large intestine, increasing peristalsis, promotes accumulation of water and electrolytes in the large intestine. This leads to stimulation of the act of defecation, reduced evacuation time and softening of stools.
Pharmacokinetics
After oral administration, sodium picosulfate passes unchanged through the stomach and small intestine, entering the colon. There is little absorption of the drug, which rules out enterohepatic circulation.
In the colon, sodium picosulfate is broken down to form the active metabolite, bis-(p- hydroxyphenyl)-pyridyl-2-methane. The time of development of the laxative effect of the drug is determined by the rate of release of the active metabolite and is 6-12 hours.
A small part of the drug enters the systemic bloodstream.
There is no correlation between the laxative effect of the active metabolite and its concentration in blood serum.
Indications
Indications
atonic constipation;
regulation of stool (hemorrhoids, proctitis, anal fissures);
preparation for surgical operations, instrumental and x-ray examinations.
Pharmacological effect
Pharmacological effect
Laxative drug. The active substance, sodium picosulfate, is a laxative of the triarylmethane group.
As a local laxative, sodium picosulfate, after bacterial breakdown in the large intestine, has a stimulating effect on the colon mucosa, increasing peristalsis, and promoting the accumulation of water and electrolytes in the large intestine. This leads to stimulation of the act of defecation, reduction of evacuation time and softening of the stool.
Pharmacokinetics
After oral administration, sodium picosulfate passes unchanged through the stomach and small intestine to enter the colon. Absorption of the drug is insignificant, which excludes its enterohepatic circulation.
In the colon, sodium picosulfate is broken down to form the active metabolite, bis-(p-hydroxyphenyl)-pyridyl-2-methane. The time for development of the laxative effect of the drug is determined by the rate of release of the active metabolite and is 6-12 hours.
A small part of the drug enters the systemic circulation.
There is no relationship between the laxative effect of the active metabolite and its concentration in the blood serum.
Special instructions
Special instructions
Do not use the drug daily without consulting a doctor for more than 10 days. Long-term use of high doses of the drug can lead to fluid loss, electrolyte imbalance, and hypokalemia.
Dizziness and fainting have been observed in patients taking Guttalax®. The analysis showed that these cases are associated with syncope with defecation (or syncope caused by straining to defecate), or with a vasovagal response to abdominal pain, which may be due to constipation, and is not necessarily related to the drug.
One tablet (5 mg) contains 67.5 mg of lactose. The maximum recommended daily dose for the treatment of adults and children over 10 years of age and for children 4-10 years of age contains 135.0 mg and 67.5 mg of lactose, respectively.
Children over 4 years of age should take the drug only as prescribed by a doctor.
Impact on the ability to drive vehicles and operate machinery
There have been no special clinical studies of the effect of the drug on the ability to drive vehicles or operate machinery. However, patients should be advised that they may experience dizziness and/or fainting due to a vasovagal reaction (i.e. during bowel spasm). If patients experience intestinal spasms, they should avoid potentially hazardous activities, incl. driving vehicles or operating machinery.
Active ingredient
Active ingredient
Sodium picosulfate
Composition
Composition
sodium picosulfate monohydrate
5.187 mg
which corresponds to the sodium picosulfate content
5 mg
Excipients:
lactose monohydrate – 71 mg,
corn starch – 41.5 mg,
colloidal silicon dioxide – 1.7 mg,
hydrolyzed potato starch – 0.3 mg,
magnesium stearate – 0.5 mg.
Pregnancy
Pregnancy
During long-term experience with the use of the drug, no adverse events were identified during pregnancy. However, due to the lack of research, the use of Guttalax® during pregnancy is recommended only in cases where the potential benefit to the mother outweighs the possible risk to the fetus. During pregnancy, the drug can be used only after consultation with a specialist.
The active metabolite and its glucuronides are not excreted in breast milk. Thus, the drug can be used during breastfeeding.
Studies on the effect of the drug on fertility have not been conducted. In preclinical studies, no teratogenic effects on reproduction were identified.
Use in children
Contraindicated in children under 4 years of age. For children from 4 to 10 years of age, use only as prescribed by a doctor.
Contraindications
Contraindications
hypersensitivity to sodium picosulfate or other ingredients of the drug;
intestinal obstruction;
strangulated hernia;
acute inflammatory diseases of the abdominal organs;
peritonitis;
abdominal pain of unknown origin;
severe dehydration;
bleeding from the gastrointestinal tract;
metrorrhagia;
cystitis;
spastic constipation;
children under 4 years of age;
pregnancy (first trimester).
Side Effects
Side Effects
From the gastrointestinal tract: discomfort, nausea, vomiting, cramps and pain in the abdomen, diarrhea.
From the nervous system: dizziness and fainting. Dizziness and fainting that occur after taking the drug appear to be associated with a vasovagal response (eg, straining during bowel movements, abdominal cramps).
From the immune system: hypersensitivity reactions, including angioedema and skin reactions.
Interaction
Interaction
Diuretics or glucocorticosteroids increase the risk of electrolyte imbalance (hypokalemia) when taking the drug in high doses.
Electrolyte imbalance may increase sensitivity to cardiac glycosides.
The combined use of the drug and antibiotics may reduce the laxative effect of the drug.
Overdose
Overdose
Symptoms
When taken in high doses, diarrhea, dehydration, decreased blood pressure, water and electrolyte imbalance, hypokalemia, and convulsions are possible.
In addition, there are reports of cases of ischemia of the muscles of the large intestine associated with taking the drug in doses significantly higher than those recommended for the usual treatment of constipation.
The drug Guttalax, like other laxatives, in chronic overdose can lead to chronic diarrhea, abdominal pain, hypokalemia, secondary hyperaldosteronism, and urolithiasis. Renal tubular damage, metabolic alkalosis, and muscle weakness associated with hypokalemia may develop due to chronic laxative abuse.
Treatment
To reduce the absorption of the drug after oral administration, you can induce vomiting or perform gastric lavage. Fluid replacement and correction of electrolyte balance may be required, as well as antispasmodics.
Storage conditions
Storage conditions
In a place protected from light, at a temperature not exceeding 25 °C
Shelf life
Shelf life
5 years
Manufacturer
Manufacturer
Delpharm Reims, France
Additional information
| Shelf life | 5 years |
|---|---|
| Conditions of storage | In a light-protected place, at a temperature not exceeding 25 °C |
| Manufacturer | Delpharm Reims, France |
| Medication form | pills |
| Brand | Delpharm Reims |
Other forms…
Related products
Buy Guttalax, tablets 5 mg 20 pcs with delivery to USA, UK, Europe and over 120 other countries.