No products in the cart.
Glucose, 400 mg/ml 10 m 10 pcs
€4.25 €3.78
Description Pharmacotherapeutic group of carbohydrate nutrition.
ATC code: B05BA03
Pharmacological properties
Pharmacodynamics
Participates in various metabolic processes in the body. Infusion of glucose solutions partially replenishes the water deficit. Dextrose entering the tissues is phosphorylated into glucose-6-phosphate, which is actively involved in many parts of the body’s metabolism.
Dextrose solution 400 mg/ml is hypertensive, increases blood osmotic pressure, dilates blood vessels, increases diuresis.
Pharmacokinetics
Glucose bioavailability after intravenous administration of 100%. Glucose is metabolized by two different pathways: anaerobic and aerobic. Dextrose breaks down into pyruvic acid or lactic acid (anaerobic glycolysis) and is metabolized to carbon dioxide and water with release of energy. Normally, glucose is absorbed completely and is not excreted by the kidneys.
Indications
Indications
– Hypoglycemia.
– As a source of carbohydrates (alone or as part of parenteral nutrition if necessary).
Pharmacological effect
Pharmacological effect
Pharmacotherapeutic group Nutrition carbohydrate drug.
ATX code: B05BA03
Pharmacological properties
Pharmacodynamics
Participates in various metabolic processes in the body. Infusion of glucose solutions partially compensates for the water deficit. Dextrose, entering the tissues, is phosphorylated, turning into glucose-6-phosphate, which is actively involved in many parts of the body’s metabolism.
A dextrose solution of 400 mg/ml is hypertonic, increases the osmotic pressure of the blood, dilates blood vessels, and increases diuresis.
Pharmacokinetics
The bioavailability of glucose after intravenous administration is 100%. Glucose is metabolized in two different ways: anaerobic and aerobic. Dextrose, breaking down into pyruvic or lactic acid (anaerobic glycolysis), is metabolized to carbon dioxide and water, releasing energy. Typically, glucose is completely absorbed and is not excreted by the kidneys.
Special instructions
Special instructions
Glucose solution should not be administered quickly or for a long time. If chills occur during the infusion of the solution, the administration should be stopped immediately. To prevent thrombophlebitis, it should be administered slowly through large veins.
For more complete and rapid absorption of dextrose, you can administer 4-5 units of short-acting insulin subcutaneously, at the rate of 1 unit of short-acting insulin per 4-5 g of dextrose.
Patient monitoring should include regular monitoring of blood glucose concentrations, fluid balance, plasma electrolyte concentrations—particularly potassium—and acid-base balance.
It is not recommended to prescribe a glucose solution in the acute period of severe traumatic brain injury, in case of acute cerebrovascular accident, since the drug can increase damage to brain structures and worsen the course of the disease (except in the case of correction of hypoglycemia).
In case of hypokalemia, the administration of a glucose solution must be combined with the correction of potassium deficiency (due to the risk of increased hypokalemia).
For hypotonic dehydration, the use of the drug is indicated simultaneously with the administration of hypertonic saline solutions.
For diabetic patients, dextrose is administered under the control of sugar levels in the blood and urine.
In case of renal failure, decompensated heart failure, hyponatremia, special caution is required when prescribing glucose, monitoring of central hemodynamic parameters is necessary.
Use in pediatrics
It is not recommended to use 40% glucose solution in doses of more than 1 ml/kg of body weight in newborns and premature infants, since there is a high risk of developing encephalopathy caused by the administration of a hypertonic solution.
Pregnancy and lactation
Use during breastfeeding, taking into account the excess of the expected benefit for the mother and the potential risk for the child, provided that the electrolyte and fluid balance are controlled and are within physiological limits. It can be used with caution if necessary, in pregnant women with reduced glucose tolerance, under the control of blood glucose levels.
Features of the effect of the drug on the ability to drive a vehicle or potentially dangerous mechanisms.
The drug does not affect the ability to drive vehicles, operate machinery, or engage in potentially hazardous activities that require increased concentration and speed of psychomotor reactions.
Active ingredient
Active ingredient
Dextrose
Composition
Composition
1 ml of solution contains
active substance
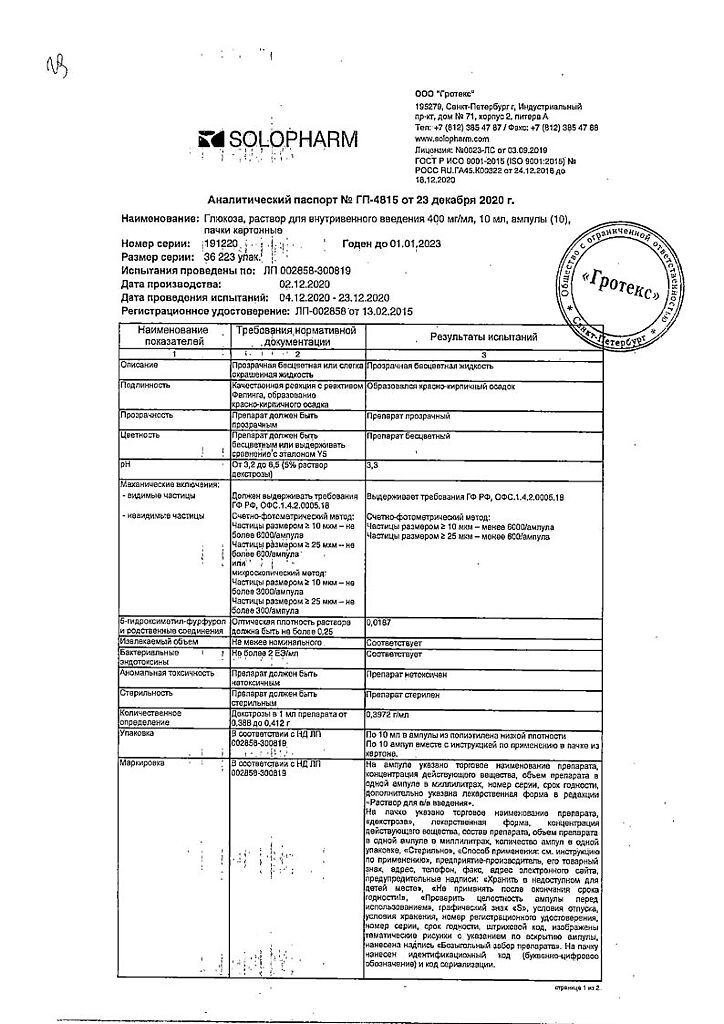
dextrose monohydrate in terms of dry matter – 400.0 mg
excipients:
hydrochloric acid 1 M solution to pH 3.0-4.0,
sodium chloride,
water for injections.
Pregnancy
Pregnancy
During pregnancy and breastfeeding, the safety and effectiveness of the drug have not been established.
The use of the drug during pregnancy and breastfeeding is possible only as prescribed by a doctor, if the expected benefit to the mother outweighs the potential risk to the fetus or child. A 40% glucose solution during pregnancy and breastfeeding can only be used under medical supervision.
Contraindications
Contraindications
Hypersensitivity to the components of the drug, hyperglycemia, hyperlactatemia, hyperhydration, postoperative disorders of glucose utilization; circulatory disorders that threaten cerebral and pulmonary edema; cerebral edema, pulmonary edema, acute left ventricular failure, hyperosmolar coma, childhood (without dilution), decompensated diabetes mellitus, dextrose intolerance, including metabolic stress.
With caution
Decompensated chronic heart failure, chronic renal failure (oligo-, anuria), hyponatremia, diabetes mellitus.
Side Effects
Side Effects
– disturbance of ion balance
– hyperglycemia
– acute left ventricular failure
– fever
– hyperglycemic hyperosmolar coma
– water-electrolyte imbalance
– with an increase in the rate of administration – osmotic diuresis with loss of water, electrolytes and the development of hyperosmolar coma
At the injection site, infection develops, sometimes thrombophlebitis
With repeated administration of the solution, disturbances in the functional state of the liver and depletion of the insular apparatus of the pancreas are possible.
Interaction
Interaction
The combined use of catecholamines and steroids reduces glucose absorption. When mixed with other drugs, they must be visually monitored for incompatibility.
In the absence of compatibility studies, the drug should not be mixed with other drugs.
Before adding any drug, you must ensure that it is soluble and stable in water within the pH range of the drug.
After adding a compatible drug to the preparation, the resulting solution should be administered immediately.
Medicines with known incompatibility should not be used.
Dextrose solutions cannot be administered through the same infusion system as for blood transfusion; there is a possible risk of hemolysis and thrombosis.
Overdose
Overdose
Symptoms: hyperglycemia, glycosuria, hyperglycemic, hyperosmolar coma, hyperhydration, water-electrolyte imbalance, fatty infiltration of the liver, acute left ventricular failure, ion imbalance.
Treatment: stop glucose administration, administer short-acting insulin, symptomatic therapy
Storage conditions
Storage conditions
Store at a temperature not exceeding 25 ºС.
Keep out of the reach of children.
Shelf life
Shelf life
2 years.
Do not use after the expiration date!
Manufacturer
Manufacturer
Grotex LLC, Russia
Additional information
| Shelf life | 2 years. Do not use after the expiration date! |
|---|---|
| Conditions of storage | Store at a temperature not exceeding 25 ºC. Keep out of reach of children. |
| Manufacturer | Grotex Ltd, Russia |
| Medication form | solution |
| Brand | Grotex Ltd |
Other forms…
Related products
Buy Glucose, 400 mg/ml 10 m 10 pcs with delivery to USA, UK, Europe and over 120 other countries.