No products in the cart.
Description
Glevo is an antibacterial drug of the group of fluoroquinolones of broad spectrum.
Levofloxacin blocks DNA-hymerase (topoisomerase II) and topoisomerase IV, disrupts superspiralization and cross-linking of DNA breaks, inhibits DNA synthesis, causes profound morphological changes in the cytoplasm, cell wall and membranes of microorganisms.
Indications
Indications
Infectious and inflammatory diseases caused by microorganisms sensitive to the drug:
infections of the ENT organs (including acute sinusitis);
infections of the lower respiratory tract (including exacerbation of chronic bronchitis, community-acquired pneumonia);
uncomplicated and complicated urinary tract and kidney infections (including acute pyelonephritis, prostatitis);
genital infections;
infections of the skin and soft tissues (festering atheromas, abscess, furunculosis);
infections of the abdominal cavity (in combination with drugs acting on anaerobic microflora).
Pharmacological effect
Pharmacological effect
Glevo is a broad-spectrum fluoroquinolone antibacterial drug.
Levofloxacin blocks DNA gyrase (topoisomerase II) and topoisomerase IV, disrupts supercoiling and cross-linking of DNA breaks, suppresses DNA synthesis, and causes profound morphological changes in the cytoplasm, cell wall and membranes of microorganisms.
Special instructions
Special instructions
During treatment, it is necessary to avoid solar and artificial UV irradiation to avoid damage to the skin (photosensitization).
Active ingredient
Active ingredient
Levofloxacin
Composition
Composition
film-coated tablets
1 tablet contains levofloxacin (in the form of hemihydrate) 500 mg
Contraindications
Contraindications
epilepsy;
tendon damage due to previous treatment with quinolones;
pregnancy;
lactation period (breastfeeding);
childhood and adolescence (up to 18 years);
hypersensitivity to levofloxacin and other fluoroquinolones.
Side Effects
Side Effects
From the digestive system: nausea, vomiting, diarrhea (including blood), indigestion, loss of appetite, abdominal pain, pseudomembranous enterocolitis, increased activity of liver transaminases, hyperbilirubinemia, hepatitis, dysbacteriosis.
From the cardiovascular system: decreased blood pressure, vascular collapse, tachycardia, prolongation of the QT interval; extremely rarely – atrial fibrillation.
From the endocrine system: hypoglycemia (increased appetite, increased sweating, trembling, nervousness).
From the central nervous system and peripheral nervous system: headache, dizziness, weakness, drowsiness, insomnia, tremor, anxiety, paresthesia, fear, hallucinations, confusion, depression, movement disorders, epileptic seizures (in predisposed patients).
From the senses: disturbances of vision, hearing, smell, taste and tactile sensitivity.
Interaction
Interaction
With simultaneous use, levofloxacin increases T1/2 of cyclosporine.
The effect of the drug is reduced by drugs that inhibit intestinal motility, sucralfate, aluminum/magnesium-containing antacid drugs and iron salts (a break of at least 2 hours between doses is required).
With simultaneous use of Glevo with NSAIDs and theophylline, convulsive readiness increases.
When using Glevo simultaneously with GCS, the risk of tendon rupture increases.
Cimetidine and drugs that block tubular secretion slow down the elimination of levofloxacin.
With the simultaneous use of hypoglycemic drugs with levofloxacin, hypo- and hyperglycemia may develop.
Therefore, strict control of blood glucose levels is necessary.
Overdose
Overdose
Symptoms: nausea, erosive lesions of the gastrointestinal mucosa, prolongation of the QT interval, confusion, dizziness, convulsions.
Treatment: symptomatic therapy is carried out, dialysis is not effective.
Storage conditions
Storage conditions
In a dry place, protected from light, at a temperature not exceeding 25 °C
Shelf life
Shelf life
2 years
Manufacturer
Manufacturer
Glenmark Pharmaceuticals Ltd, India
Additional information
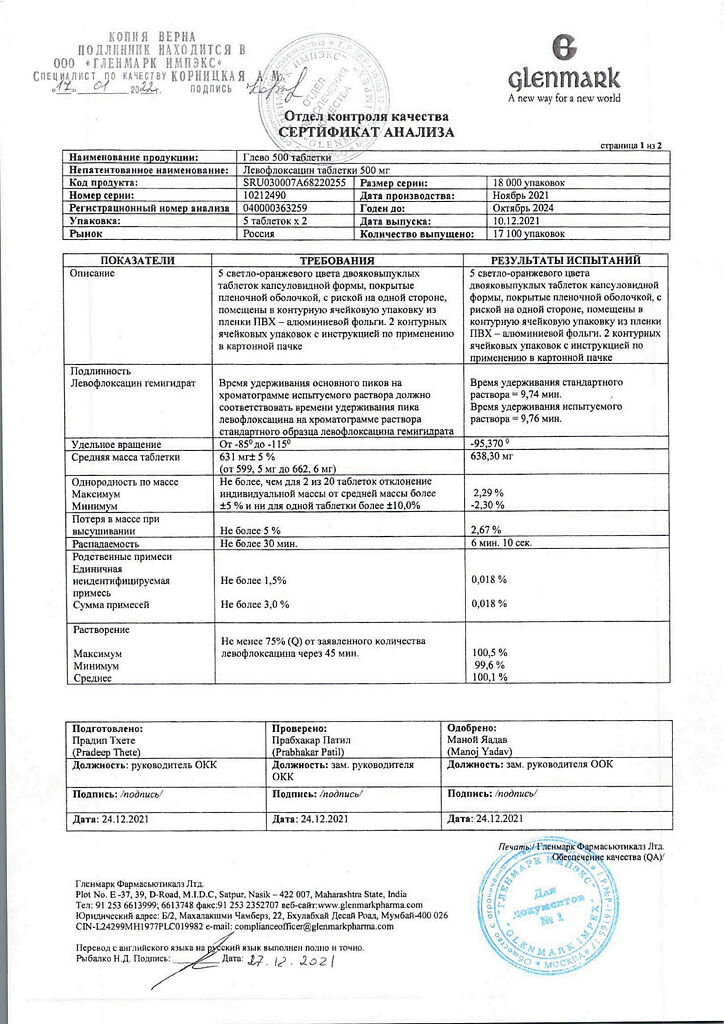
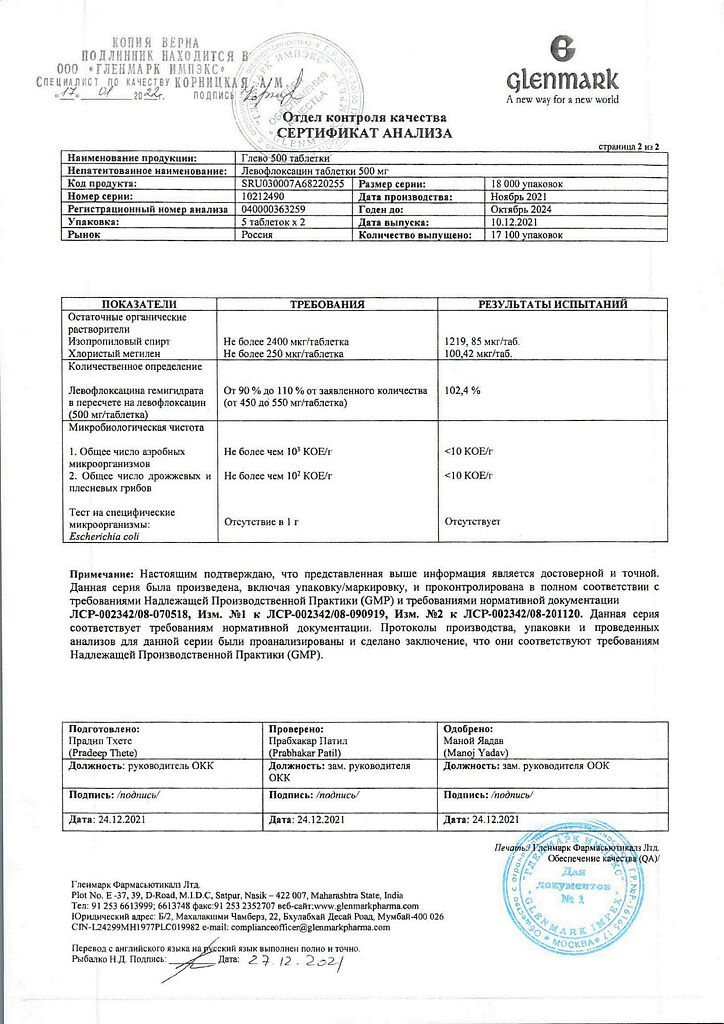
| Shelf life | 2 years |
|---|---|
| Conditions of storage | In a dry, light-protected place at a temperature not exceeding 25 °C |
| Manufacturer | Glenmark Pharmaceuticals Ltd, India |
| Medication form | pills |
| Brand | Glenmark Pharmaceuticals Ltd |
Other forms…
Related products
Buy Glevo, 500 mg 10 pcs with delivery to USA, UK, Europe and over 120 other countries.