No products in the cart.
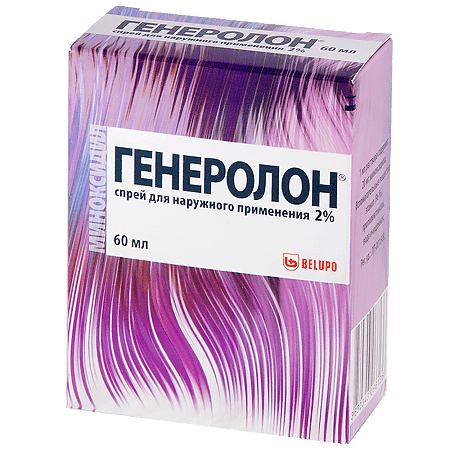
Generolon, 2% spray 60 ml
€25.10 €20.92
Description
Generolon – stimulates hair growth.
Pharmacodynamics
When applied topically, minoxidil stimulates hair growth in persons with androgenic alopecia (hair thinning, baldness). It improves microcirculation, stimulates the transition of hair cells into an active growing phase, changes the effect of androgens on the hair follicles. It reduces the formation of 5-alpha-dehydrotestosterone (possibly indirectly), which plays a significant role in the formation of baldness.
The best effect is achieved if the time of the disease is short (not more than 10 years), the patient is young, the baldness in the vertex is not more than 10 cm, the presence of more than 100 down and terminal hairs in the center of the baldness. The manifestation of signs of hair growth is observed after 4 months or more of using the drug.
The beginning and degree of the effect may vary from patient to patient. The 5% solution of the drug stimulates hair growth more strongly than the 2% solution, which has been noted in increased growth of downy hair.
After discontinuation of Generolon® the growth of new hair is suspended and within 3-4 months it is possible to restore the initial appearance. The exact mechanism of action of Generolone® in treatment of androgenic alopecia is unknown. Minoxidil is ineffective in cases of alopecia caused by taking drugs, improper diet (iron deficiency, vitamin A deficiency), as a result of hair styling in tight hairstyles.
Pharmacokinetics
In external application, minoxidil is poorly absorbed through normal intact skin: on average, 1.5% (0.3-4.5%) of the total dose applied enters the systemic bloodstream. The effect of concomitant skin diseases on the absorption of minoxidil is unknown. After discontinuation of the drug, approximately 95% of minoxidil entering the systemic bloodstream is eliminated within 4 days.
The metabolic biotransformation profile of minoxidil after external use of Generolone® has not yet been fully understood.
Minoxidil does not bind to plasma proteins and is excreted by the kidneys via glomerular filtration. Minoxidil does not penetrate the HEB.
It is excreted mainly in the urine. Minoxidil and its metabolites are excreted by hemodialysis.
Indications
Indications
Treatment of androgenetic alopecia (hair restoration) and stabilization of hair loss in men and women.
Pharmacological effect
Pharmacological effect
Generolon – stimulating hair growth.
Pharmacodynamics
When applied topically, minoxidil stimulates hair growth in people with androgenetic alopecia (hair thinning, baldness). Improves microcirculation, stimulates the transition of hair cells into the active growing phase, changes the effect of androgens on hair follicles. Reduces the formation of 5-alpha-dehydrotestosterone (possibly indirectly), which plays a significant role in the formation of baldness.
The best effect is achieved when the disease is not long ago (no more than 10 years), the patients are young, the bald spot in the crown area is no more than 10 cm, and there are more than 100 vellus and terminal hairs in the center of the bald spot. Signs of hair growth appear after 4 or more months of using the drug.
The onset and severity of the effect may vary from patient to patient. A 5% solution of the drug stimulates hair growth more than a 2% solution, which was noted by an increase in the growth of vellus hair.
After stopping the use of the drug Generalolon®, the growth of new hair stops, and within 3–4 months the original appearance can be restored. The exact mechanism of action of the drug Generalolon® in the treatment of androgenetic alopecia is unknown. Minoxidil is ineffective in case of baldness caused by taking drugs, poor diet (iron deficiency, vitamin A), as a result of styling hair in tight hairstyles.
Pharmacokinetics
When applied topically, minoxidil is poorly absorbed through normal intact skin: on average, 1.5% (0.3–4.5%) of the total applied dose enters the systemic circulation. The effect of concomitant skin diseases on the absorption of minoxidil is unknown. After stopping use of the drug, approximately 95% of minoxidil that enters the systemic circulation is eliminated within 4 days.
The profile of the metabolic biotransformation of minoxidil after external use of the drug Generolon® has not yet been fully studied.
Minoxidil does not bind to plasma proteins and is excreted by the kidneys through glomerular filtration. Minoxidil does not penetrate the BBB.
Excreted mainly in urine. Minoxidil and its metabolites are eliminated by hemodialysis.
Special instructions
Special instructions
Do not apply the drug to other parts of the body.
The drug Generalolon® should be applied only to dry skin of the scalp after bathing, and after applying the drug you should wait about 4 hours before bathing (do not wet your hair earlier than 4 hours after applying the drug).
When using the drug Generalolon®, it is recommended to wash your hair as usual. During the period of use of the drug Generalolon®, you can use hairspray and other hair care products. Before applying hair care products, you must first apply Generolon® and wait until the treated area of skin is completely dry. There is no evidence that hair coloring, perming, or using hair softeners may reduce the effectiveness of the drug. However, to prevent possible scalp irritation, you must ensure that the product is completely rinsed from the hair and scalp before using these chemicals.
Before starting treatment with Generolon®, patients should undergo a general examination, including the collection and study of a medical history. The doctor must make sure that the scalp is healthy.
If systemic side effects or severe skin reactions occur, patients should discontinue the drug and consult a doctor.
Generolon® contains ethyl alcohol, which can cause inflammation and irritation of the eyes. If the drug gets on sensitive surfaces (eyes, irritated skin, mucous membranes), it is necessary to rinse the area with plenty of cold water. Avoid inhalation of the drug when spraying. After using the drug, wash your hands thoroughly.
Active ingredient
Active ingredient
Minoxidil
Composition
Composition
Active ingredient:
minoxidil 20 mg;
Excipients:
ethanol (96%) – 571 mg;
propylene glycol – 104 mg;
water – up to 1 ml
Pregnancy
Pregnancy
The drug should not be used during pregnancy and breastfeeding.
Contraindications
Contraindications
Hypersensitivity to minoxidil or other components of the drug;
age under 18 years;
violation of the integrity of the skin;
dermatoses of the scalp.
With caution: elderly people (over 65 years).
Side Effects
Side Effects
Local side effects
The most common side effect is scalp dermatitis. Less common are more severe cases of dermatitis, which manifest as redness, peeling and inflammation. In rare cases, scalp itching, allergic contact dermatitis, folliculitis, hypertrichosis (unwanted body hair growth, including facial hair growth in women), and seborrhea are noted.
The use of minoxidil may cause increased hair loss during the transition from the resting phase to the hair growth phase, with old hair falling out and new hair growing in its place. This temporary phenomenon is usually observed 2-6 weeks after the start of treatment and gradually, over the next 2 weeks, stops (the first signs of the action of minoxidil appear).
Systemic side effects (in case of accidental ingestion of the drug)
Skin and subcutaneous tissue: nonspecific allergic reactions (skin rash, urticaria), facial swelling.
Respiratory system: shortness of breath, allergic rhinitis.
Nervous system: headache, dizziness, vertigo, neuritis.
CVS: chest pain, blood pressure fluctuations, rapid heartbeat, changes in heart rate, edema.
Interaction
Interaction
There is a theoretical possibility (no clinical evidence) of increased orthostatic hypotension in patients receiving concomitant treatment with peripheral vasodilators.
A very slight increase in the content of minoxidil in the blood of patients suffering from arterial hypertension and taking minoxidil orally cannot be excluded in the case of simultaneous use of the drug Generolon®, although corresponding clinical studies have not been conducted.
It has been established that minoxidil for external use can interact with some drugs for external use. The simultaneous use of minoxidil solution for external use and a cream containing betamethasone (0.05%) leads to a decrease in the systemic absorption of minoxidil.
Concomitant use of a cream containing tretinoin (0.05%) leads to increased absorption of minoxidil. Simultaneous application of minoxidil to the skin and topical drugs such as tretinoin and dithranol, which cause changes in the protective functions of the skin, may lead to increased absorption of minoxidil.
Overdose
Overdose
Accidental ingestion of the drug Generolon can cause systemic side effects due to the vasodilatory properties of minoxidil (5 ml of a 2% solution contains 100 mg of minoxidil – the maximum recommended dose for adults when taken orally in the treatment of arterial hypertension; 5 ml of a 5% solution contains 250 mg of minoxidil, that is, a dose 2.5 times higher than the maximum recommended daily dose for adults for taken orally in the treatment of arterial hypertension).
Signs of overdose: fluid retention, decreased blood pressure, tachycardia.
Treatment: to eliminate fluid retention, diuretics can be prescribed if necessary; for the treatment of tachycardia – beta-adren about locators. To treat gynotension, 0.9% sodium chloride solution should be administered intravenously.
Symiatomimetic drugs, such as norepinsphrine and epinsphrine, which have excessive cardiac stimulating activity, should not be prescribed.
Storage conditions
Storage conditions
At a temperature not exceeding 25 °C
Shelf life
Shelf life
3 years
Manufacturer
Manufacturer
Industrial Pharmaceutical Cantabria S.A., Spain
Additional information
| Shelf life | 3 years |
|---|---|
| Conditions of storage | At a temperature not exceeding 25 °C |
| Manufacturer | Industrial Pharmacy Cantabria S.A., Spain |
| Medication form | topical spray |
| Brand | Industrial Pharmacy Cantabria S.A. |
Related products
Buy Generolon, 2% spray 60 ml with delivery to USA, UK, Europe and over 120 other countries.