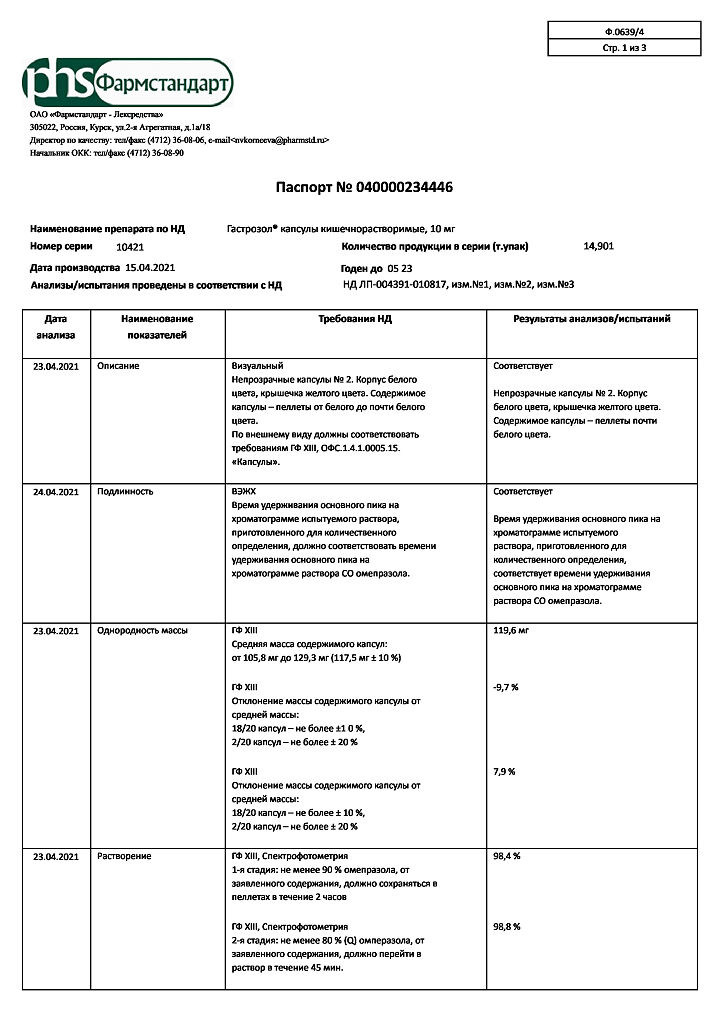
No products in the cart.
Gastrozol, 10 mg capsules 14 pcs
€3.92 €3.56
EAN: 4601669011916
SKU: 327656
Categories: Medicine, Stomach, intestines, liver, Ulcer and gastritis
Description
Pharmacotherapeutic group: gastric gland secretion reducing agent – proton pump inhibitor
ATX code: A02BC01
Pharmacological action
Pharmacodynamics
Omeprazole inhibits the enzyme H+K+-ATPase (“proton pump”) in the parietal cells of the stomach, thereby blocking the final stage of hydrochloric acid secretion. This leads to a decrease in basal and stimulated acid secretion regardless of the nature of the stimulus.
After a single oral dose, the effects of omeprazole occur within the first hour and last for 24 hours. Maximum effect is reached after 2 hours. After discontinuation of the drug secretory activity is fully restored in 3 to 5 days.
Pharmacokinetics
Intake
After oral administration, omeprazole is rapidly absorbed from the gastrointestinal tract, the maximum concentration (Cmax) of omeprazole in plasma is reached after 0.5 to 3.5 hours. Bioavailability is 30-40%.
Distribution
The binding to plasma proteins is about 95%.
Metabolism
Omeprazole is almost completely metabolized in the liver with participation of CYP2C19 isoenzyme to form six pharmacologically inactive metabolites.
The elimination half-life (T1/2) is 0 5 – 1 hour. It is excreted as metabolites by kidneys (70 – 80%) and with bile (20 – 30%).
Pharmacokinetics in special clinical cases
In patients with hepatic insufficiency the bioavailability is significantly increased and the elimination half-life is increased up to 3 hours. In elderly patients the elimination rate decreases and the bioavailability increases.
Indications
Indications
Symptoms of gastroesophageal reflux such as heartburn, sour belching.
Pharmacological effect
Pharmacological effect
Pharmacotherapeutic group: agent that reduces the secretion of gastric glands – proton pump inhibitor
ATX code: A02BC01
Pharmacological action
Pharmacodynamics
Omeprazole inhibits the enzyme H+K+-ATPase (“proton pump”) in the parietal cells of the stomach, thereby blocking the final stage of hydrochloric acid secretion. This leads to a decrease in the level of basal and stimulated acid secretion, regardless of the nature of the stimulus.
After a single oral dose, the effect of omeprazole occurs within the first hour and continues for 24 hours. The maximum effect is achieved after 2 hours. After stopping the drug, secretory activity is completely restored after 3 to 5 days.
Pharmacokinetics
Suction
After oral administration, omeprazole is rapidly absorbed from the gastrointestinal tract, the maximum concentration (Cmax) of omeprazole in the blood plasma is reached after 0.5 – 3.5 hours. Bioavailability is 30-40%.
Distribution
Binding to blood plasma proteins is about 95%.
Metabolism
Omeprazole is almost completely metabolized in the liver with the participation of the CYP2C19 isoenzyme with the formation of six pharmacologically inactive metabolites.
Removal
The half-life (T1/2) is 0 5 – 1 hour. Excreted in the form of metabolites by the kidneys (70 – 80%) and with bile (20 – 30%).
Pharmacokinetics in special clinical situations
In patients with hepatic impairment, bioavailability increases significantly and the half-life increases to 3 hours. In elderly patients, the rate of elimination decreases and bioavailability increases.
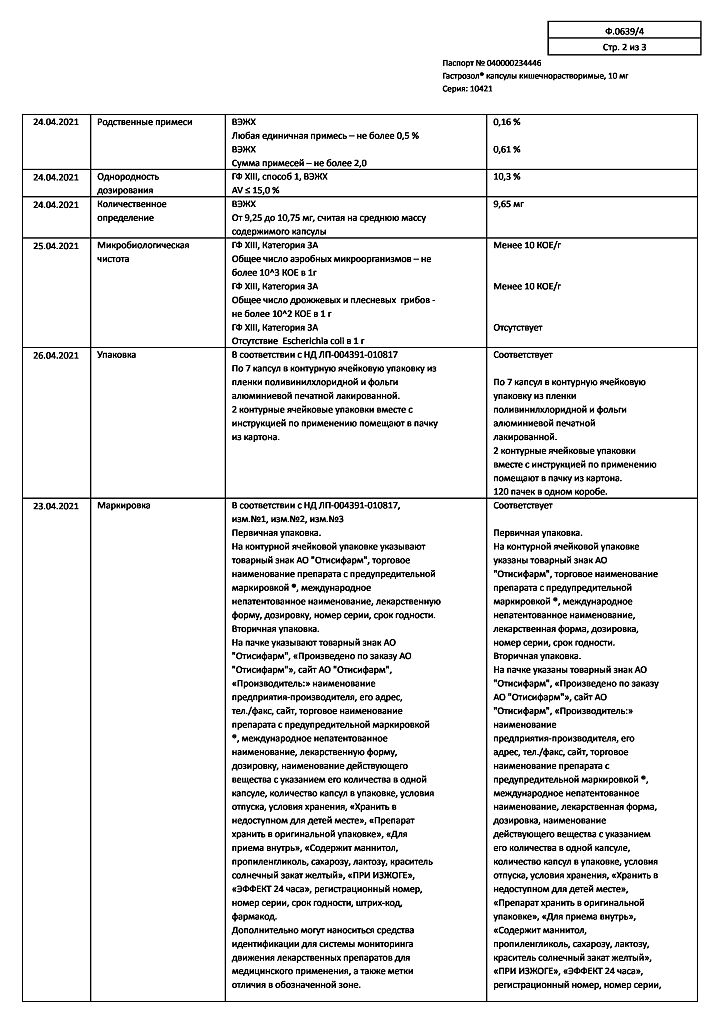
Special instructions
Special instructions
Omeprazole is not intended for use in cases of occasional heartburn (heartburn less than 2 times a week).
Before starting therapy with omeprazole, it is necessary to exclude the presence of a malignant process in the upper gastrointestinal tract, since taking omeprazole can mask symptoms and delay the correct diagnosis.
A decrease in the secretion of hydrochloric acid in the stomach under the influence of proton pump inhibitors leads to an increase in the growth of normal intestinal microflora and may lead to a slight increase in the risk of developing intestinal infections caused by bacteria of the genus Salmonella spp., Campylobacter spp., as well as Clostridium difficile bacteria.
A decrease in the secretion of hydrochloric acid can lead to an increase in the concentration of chromogranin A (CgA), which affects the results of examinations for the detection of neuroendocrine tumors, therefore, to prevent this effect, it is necessary to temporarily stop taking omeprazole 5 days before the CgA concentration test. If during this time the CgA concentration has not returned to normal, the study should be repeated.
The risk-benefit ratio of long-term (more than 1 year) omeprazole maintenance therapy should be regularly assessed. There is evidence of an increased risk of fractures of the vertebrae, wrist bones, and head of the femur, mainly in elderly patients or in the presence of other risk factors. Patients at risk of developing osteoporosis should ensure adequate intake of vitamin D and calcium.
Severe hypomagnesemia, manifested by symptoms such as fatigue, delirium, seizures, dizziness and ventricular arrhythmia, has been reported in patients receiving omeprazole for at least three months. In most patients, hypomagnesemia was relieved after discontinuation of proton pump inhibitors and administration of magnesium supplements.
Patients receiving omeprazole therapy for a long time, especially in combination with digoxin or other drugs that reduce magnesium levels in the blood plasma (diuretics), require regular monitoring of magnesium levels.
Omeprazole, like all drugs that reduce acidity, can lead to decreased absorption of vitamin B12 (cyanocobalamin). This must be remembered in patients with a reduced supply of vitamin B12 during long-term therapy.
In patients taking drugs orally for a long time that reduce the secretion of gastric glands, the formation of glandular cysts in the stomach was more often observed. These phenomena are caused by physiological changes as a result of inhibition of hydrochloric acid secretion, and undergo reverse development as therapy continues.
Impact on the ability to drive vehicles and machinery
During treatment with omeprazole, dizziness and confusion may occur.
consciousness, drowsiness, blurred vision, so caution should be exercised
when driving vehicles or operating other mechanisms and performing
other potentially hazardous activities requiring increased
concentration and speed of psychomotor reactions.
Active ingredient
Active ingredient
Omeprazole
Composition
Composition
Omeprazole pellets – 117.5 mg, containing:
active ingredient: omeprazole – 10.0 mg;
excipients: mannitol – 19.97 mg, sucrose – 32.11 mg, disodium hydrogen phosphate – 1.49 mg, sodium lauryl sulfate – 0.40 mg, lactose monohydrate – 4.00 mg, calcium carbonate – 4.00 mg, hypromellose (hydroxypropyl methylcellulose E-5) – 10.28 mg, methacrylic acid and ethyl acrylate copolymer [1:1](L-30D) – 29.37 mg, propylene glycol – 0.95 mg, diethyl phthalate – 2.94 mg, cetyl alcohol – 0.88 mg, sodium hydroxide – 0.18 mg, polysorbate-80 – 0.35 mg, povidone (polyvinylpyrrolidone, povidone K-30) – 0.30 mg, titanium dioxide – 0.21, talc – 0.07 mg.
Composition of the hard gelatin capsule shell:
body: titanium dioxide (E 171) – 2.0000%, gelatin – up to 100%.
cap: titanium dioxide (E171) – 1.3333%, sunset yellow dye (E 110) – 0.0044%, quinoline yellow (E 104) – 0.9197%, gelatin – up to 100%.
Pregnancy
Pregnancy
The results of three prospective epidemiological studies (more than 1000 observations) showed that the use of omeprazole in pregnant women does not have a negative effect on the course of pregnancy and the health of the fetus/newborn. However, before using the drug during pregnancy, it is recommended to consult a doctor.
Omeprazole passes into breast milk. If necessary, use the drug
During lactation, the issue of stopping breastfeeding should be decided.
Contraindications
Contraindications
Hypersensitivity to omeprazole, substituted benzimidazoles or other components of the drug.
Concomitant use with nelfinavir, atazanavir, erlotinib, posaconazole.
Age up to 18 years.
Precautions for use
Before using the drug, you should consult your doctor in the following cases:
– in the presence of previously diagnosed gastric ulcer; severe liver disease accompanied by liver failure; jaundice; previous surgical intervention on the gastrointestinal tract;
– in the presence of “alarming” symptoms: significant spontaneous loss of body weight, repeated vomiting, vomiting mixed with blood, changes in the color of stool (tarry stools – melena), difficulty swallowing;
– when new symptoms appear or changes in existing symptoms from the gastrointestinal tract;
– in the presence of osteoporosis;
– against the background of ongoing symptomatic treatment for indigestion or persistent heartburn (for 4 weeks or more);
– when used simultaneously with one or more of the following drugs: clopidogrel, digoxin, erlotinib, ketoconazole, itraconazole, warfarin, cilostazol, diazepam, phenytoin, saquinavir, tacrolimus, clarithromycin, voriconazole, rifampicin, St. John’s wort preparations (see section Interactions with other drugs drugs).
It is also recommended to consult a doctor before using Gastrozol® during pregnancy and breastfeeding (see section Use during pregnancy and breastfeeding.)
Side Effects
Side Effects
The frequency of side effects is presented in accordance with the following gradation: very often (≥1/10); often (≥1/100, <1/10); uncommon (≥1/1000, <1/100); rare (≥1/10000, <1/1000); very rare (<1/10000).
Allergic reactions: uncommon – urticaria, skin rash, itching; rarely – fever, urticaria, angioedema, anaphylactoid reactions; very rarely – eosinophilia.
From the gastrointestinal tract: often – nausea, vomiting, abdominal pain, diarrhea or constipation, flatulence; infrequently – increased activity of liver enzymes (transaminases) and alkaline phosphatase (reversible), taste distortion (usually resolves after cessation of therapy); rarely – dry mouth, stomatitis, candidiasis of the gastrointestinal tract, hepatitis (with or without jaundice), microscopic colitis, discoloration of the tongue to brown-black and the appearance of benign cysts of the salivary glands when used simultaneously with clarithromycin (the phenomena are reversible after cessation of therapy); very rarely – liver failure (in patients with previous severe liver disease).
From the nervous system: often – headache; infrequently – dizziness, paresthesia, drowsiness, insomnia; rarely – agitation, reversible confusion, depression; very rarely – aggression, confusion, hallucinations; against the background of severe liver disease – encephalopathy.
From the skin and subcutaneous tissues: infrequently – dermatitis; rarely – photosensitivity (redness of the skin), alopecia; very rarely – exudative erythema multiforme, Stevens-Johnson syndrome (severe erythema multiforme, characterized by the appearance of spots and blisters on the skin and mucous membranes against the background of high fever and joint pain), toxic epidermal necrolysis; frequency unknown – subacute cutaneous lupus erythematosus.
On the part of the organ of vision: infrequently – visual disturbances, including a decrease in visual fields, a decrease in the acuity and clarity of visual perception (usually disappear after cessation of therapy).
From the organ of hearing and labyrinthine disorders: infrequently – disorders of auditory perception, including “ringing in the ears” (usually disappear after cessation of therapy), vertigo.
From the musculoskeletal and connective tissue side: infrequently – fractures of the vertebrae, wrist bones, femoral head (see section “Special instructions”); rarely – myalgia (muscle pain), arthralgia (joint pain), muscle weakness.
From the respiratory system: rarely – bronchospasm.
From the kidneys and urinary tract: rarely – interstitial nephritis.
From the hematopoietic organs: rarely – leukopenia, thrombocytopenia; very rarely – agranulocytosis, pancytopenia.
From the endocrine system: very rarely – gynecomastia.
Other: infrequently – peripheral edema, malaise; rarely – increased sweating, hyponatremia; very rarely – hypomagnesemia, hypocalcemia due to severe hypomagnesemia.
Cases of the formation of glandular cysts in the stomach have been reported in patients taking drugs that reduce the secretion of gastric glands for a long period of time; The cysts are benign and go away on their own with continued therapy.
If side effects not listed in this instruction occur,
you must consult a doctor immediately.
Interaction
Interaction
You should consult your doctor before taking Gastrozol® if you are being treated with one or more of the drugs listed in this section.
A decrease in the secretion of hydrochloric acid in the stomach during treatment with omeprazole and other proton pump inhibitors can lead to a decrease or increase in the absorption of other drugs, the absorption of which depends on the acidity of the environment.
Like other drugs that reduce gastric acidity, treatment with omeprazole may lead to decreased absorption of ketoconazole, itraconazole, posaconazole, erlotinib, iron supplements and cyanocobalamin. Their co-administration with omeprazole should be avoided.
The bioavailability of digoxin when used simultaneously with omeprazole increases by 10% (adjustment of the digoxin dosage regimen may be required). Caution should be exercised when these drugs are used concomitantly in elderly patients.
Omeprazole has been shown to interact with some antiretroviral drugs. The mechanisms and clinical significance of these interactions are not always known. An increase in pH during omeprazole therapy may affect the absorption of antiretroviral drugs.
Interaction at the level of the CYP2C19 isoenzyme is also possible. When omeprazole is co-administered with certain antiretroviral drugs, such as atazanavir and nelfinavir, a decrease in their serum concentrations is observed during omeprazole therapy.
When used simultaneously with omeprazole, the area under the concentration-time curve of atazanavir decreases by 75%. In this regard, the combined use of omeprazole with antiretroviral drugs such as atazanavir and nelfinavir is contraindicated.
When used simultaneously with omeprazole, an increase in plasma concentrations of saquinavir / ritonavir is observed up to 70%, while the tolerability of treatment in patients with HIV infection does not deteriorate.
With simultaneous use of omeprazole with clopidogrel, a decrease in the antiplatelet effect of the latter is observed.
When used simultaneously with omeprazole, it is possible to increase the plasma concentration and increase the half-life of warfarin, diazepam, phenytoin, cilostazol, imipramine, clomipramine, citalopram, hexobarbital, disulfiram, as well as other drugs metabolized in the liver with the participation of the CYP2C19 isoenzyme (a dose reduction of these drugs may be required).
With simultaneous use of omeprazole and tacrolimus, an increase in the concentration of tacrolimus in the blood serum was noted, which may require dose adjustment.
In some patients, an increase in methotrexate concentrations was observed during combined use with proton pump inhibitors. When prescribing high doses of methotrexate, the possibility of temporary withdrawal of omeprazole should be considered.
Concomitant use with inhibitors of the CYP2C19 and CYP3A4 isoenzymes (such as clarithromycin and voriconazole) may lead to an increase in the plasma concentration of omeprazole, which may require dose adjustment of omeprazole in patients with severe liver failure, in case of long-term use.
Inducers of the CYP2C19 and CYP3A4 isoenzymes (for example, rifampicin, preparations of St. John’s wort (Hypericum perforatum), when used together with omeprazole, can accelerate its metabolism, thereby reducing the concentration of omeprazole in the blood plasma.
No clinically significant interaction of omeprazole with antacids, caffeine, propranolol, theophylline, metoprolol, lidocaine, quinidine, erythromycin, phenacetin, estradiol, amoxicillin, budesonide, diclofenac, metronidazole, naproxen, piroxicam has been established.
Overdose
Overdose
Symptoms: dizziness, confusion, apathy, depression, drowsiness, headache, blurred vision, vascular dilatation, tachycardia, nausea, vomiting, flatulence, abdominal pain, diarrhea, increased sweating, dry mouth.
Treatment: symptomatic. If necessary, gastric lavage,
purpose of activated carbon. Hemodialysis is not effective enough.
Storage conditions
Storage conditions
Store at a temperature not exceeding 25 ºС.
Keep out of the reach of children.
Shelf life
Shelf life
2 years.
Manufacturer
Manufacturer
Pharmstandard-Leksredstva, Russia
Additional information
| Shelf life | 2 years. |
|---|---|
| Conditions of storage | Store at a temperature not exceeding 25 ºC. Contain out of reach of children. |
| Manufacturer | Pharmstandard-Leksredstva, Russia |
| Medication form | capsules |
| Brand | Pharmstandard-Leksredstva |
Related products
Buy Gastrozol, 10 mg capsules 14 pcs with delivery to USA, UK, Europe and over 120 other countries.