No products in the cart.
Freenosol, spray 15 ml
€8.62 €7.19
Description
A combined anti-allergic agent (H1-histamine receptor blocker + alpha-adrenomimetic).
ATX code
R01AB01
Pharmacological action
/p>
Pharmacodynamics
Pharmacodynamics
Combined drug for local intranasal use for pathogenetic and symptomatic treatment with vasoconstrictor and anti-allergic action.
Phenylephrine is an alpha 1-adrenergic receptor agonist (sympathomimetic), causes narrowing of blood vessels of the nasal cavity mucosa, reduces mucosal edema and tissue hyperemia, congestion in the nasal mucosa. Restoration of nasopharyngeal air permeability improves the patient’s well-being and reduces the risk of possible complications caused by mucous secretion stasis.
Cetirizine is a blocker of H1-histamine receptors, it has anti-allergic activity, reduces capillary permeability, prevents development of edema of the mucous membrane of the nasal cavity and its accessory sinuses.
The drug contains an auxiliary humectant glycerol which promotes moisture retention and helps provide hydration in case of dryness and irritation of the nasal mucosa.
Pharmacokinetics
The drug is intended for topical intranasal use and its activity is independent of plasma concentrations of active ingredients.
Indications
Indications
acute rhinitis (including colds);
allergic rhinitis (including hay fever);
vasomotor rhinitis;
chronic rhinitis;
acute and chronic sinusitis;
acute otitis media (as an auxiliary treatment method);
preparation for surgical interventions in the nasal area and elimination of swelling of the mucous membrane of the nasal cavity and paranasal sinuses after surgical interventions in this area.
Pharmacological effect
Pharmacological effect
Combined antiallergic agent (H1-histamine receptor blocker + alpha-adrenergic agonist).
ATX code
R01AB01
Pharmacological action
Pharmacodynamics
Pharmacodynamics
A combined drug for local intranasal use for pathogenetic and symptomatic treatment, which has a vasoconstrictor and antiallergic effect.
Phenylephrine is an alpha-1-adrenergic receptor agonist (sympathomimetic), causes constriction of the blood vessels of the nasal mucosa, reduces swelling of the mucous membranes and tissue hyperemia, congestion in the nasal mucosa. Restoring air patency of the nasopharynx improves the patient’s well-being and reduces the risk of possible complications caused by stagnation of mucous secretion.
Cetirizine is a blocker of H1-histamine receptors, has an antiallergic effect, reduces capillary permeability, and prevents the development of edema of the nasal mucosa and paranasal sinuses.
The drug contains an auxiliary humectant glycerol, which promotes moisture retention, which helps provide hydration for dry and irritated nasal mucosa.
Pharmacokinetics
The drug is intended for local intranasal use, and its activity does not depend on the concentration of active substances in the blood plasma.
Special instructions
Special instructions
The drug should not be used continuously for more than 7 days. Prolonged or excessive use of the drug can cause tachyphylaxis and the “ricochet” effect associated with the re-development of nasal congestion (rhinitis medicamentosa), leading to the development of a systemic vasoconstrictor effect.
As with the use of other vasoconstrictors, the recommended dosage of the drug should not be exceeded. Otherwise, manifestations of the systemic effect of the drug may develop, especially in elderly patients.
Phenylephrine should not be used in patients within 2 weeks of discontinuation of monoamine oxidase inhibitors as they may enhance the adrenergic effects of sympathomimetic agents and increase the risk of cardiovascular side effects.
The drug is intended for local intranasal use only.
Impact on the ability to drive vehicles and machinery
Caution should be exercised when driving vehicles and machinery, as well as performing other activities that require concentration and speed of psychomotor reactions, since when using the drug there is a risk of dizziness.
Active ingredient
Active ingredient
Phenylephrine, Cetirizine
Composition
Composition
1 ml of the drug contains:
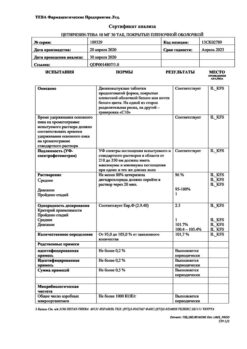
active ingredients: phenylephrine hydrochloride – 2.5 mg, cetirizine dihydrochloride – 2.5 mg;
excipients: glycerol – 40.0 mg, sodium hydrogen phosphate (anhydrous) – 2.5 mg, potassium dihydrogen phosphate – 1.5 mg, disodium edetate – 0.2 mg, benzalkonium chloride solution 50% – 0.1 mg in terms of 100%, purified water – up to 1 ml.
Pregnancy
Pregnancy
Due to the lack of data, the use of the drug is contraindicated during pregnancy and breastfeeding.
Contraindications
Contraindications
hypersensitivity to phenylephrine, cetirizine or other components of the drug;
atrophic rhinitis;
taking monoamine oxidase inhibitors (simultaneously or in the previous 14 days);
angle-closure glaucoma;
children under 18 years of age (data on effectiveness and safety in children are not available);
pregnancy, breastfeeding period.
With caution
cardiovascular diseases, including arterial hypertension, arrhythmias, generalized atherosclerosis;
hyperthyroidism;
prostate adenoma;
chronic renal failure;
old age;
patients with increased seizure activity;
angina pectoris;
diabetes mellitus;
obstruction of the bladder neck (for example, due to prostatic hypertrophy);
epilepsy;
patients with severe reactions to sympathomimetics, manifested in the form of insomnia, dizziness, tremor, cardiac arrhythmia or increased blood pressure (as is the case with the use of any local vasoconstrictor).
Side Effects
Side Effects
Cetirizine in combination with phenylephrine in the form of a spray in therapeutic doses is well tolerated, but the following side effects may be observed.
Classification of the incidence of side effects according to the recommendations of the World Health Organization (WHO):
very often ≥ 1/10;
often from ≥ 1/100 to < 1/10;
infrequently from ≥ 1/1000 to < 1/100;
rarely from ≥ 1/10000 to < 1/1000;
very rarely <1/10000, including isolated reports;
frequency unknown – based on available data, it is not possible to determine the frequency of occurrence.
Blood and lymphatic system disorders:
very rarely: thrombocytopenia.
Immune system disorders:
rarely: hypersensitivity reactions;
very rare: anaphylactic shock.
Metabolic and nutritional disorders:
frequency unknown – increased appetite.
Mental disorders:
infrequently – excitement;
rarely – aggression, confusion, depression, hallucinations, sleep disturbance;
very rarely – tic;
frequency unknown – suicidal ideation, sleep disturbances (including nightmares).
Nervous system disorders:
infrequently – paresthesia;
rarely – convulsions;
very rarely – taste perversion, dyskinesia, dystonia, fainting, tremor;
frequency unknown – headache, dizziness, memory impairment, incl. amnesia, deafness.
Visual disorders:
very rarely – disturbance of accommodation, blurred vision, nystagmus;
frequency unknown – vasculitis.
Disorders of the hearing organ and labyrinth:
frequency unknown – vertigo.
Cardiac disorders:
rarely – tachycardia;
frequency unknown – arrhythmia.
Vascular disorders:
frequency unknown – increased blood pressure.
Gastrointestinal disorders:
infrequently – diarrhea.
Disorders of the liver and biliary tract:
rarely – liver failure with changes in liver function tests (increased activity of transaminases, alkaline phosphatase, gamma-glutamyltransferase and bilirubin).
Disorders of the skin and subcutaneous tissues:
uncommon – rash, itching;
rarely – urticaria;
very rarely – angioedema, persistent drug erythema.
Muscle, skeletal and connective tissue disorders:
frequency unknown: arthralgia.
Renal and urinary tract disorders:
very rarely: dysuria;
frequency unknown: urinary retention.
General disorders and reactions at the injection site:
infrequently – asthenia, malaise;
rarely – discomfort in the nose, dryness or burning in the nose, tingling, sneezing; nosebleed. Benzalkonium chloride, which is part of the drug, may cause irritation of the nasal mucosa;
frequency unknown – swelling and hyperemia of the nasal mucosa, mucous and watery discharge from the nose, difficulty in nasal breathing.
Interaction
Interaction
The drug should not be used simultaneously with tri- and tetracyclic antidepressants, beta-blockers, monoamine oxidase inhibitors (procarbazine, selegiline), maprotiline, guanedrel, guanethidine.
Thyroid hormones with systemic absorption of phenylephrine increase the (mutually) associated risk of coronary insufficiency (especially with coronary atherosclerosis).
Overdose
Overdose
The extremely low systemic absorption of the drug when applied topically makes overdose almost impossible.
Symptoms
With an accidental single ingestion of cetirizine at a dose of 50 mg, the following may be observed: confusion, diarrhea, dizziness, fatigue, headache, malaise, mydriasis, itching, anxiety, weakness, sedation, drowsiness, stupor, tachycardia, tremor, urinary retention.
An overdose of phenylephrine can be manifested by heart rhythm disturbances, increased blood pressure, and agitation.
Treatment
Gastric lavage or stimulation of vomiting, administration of activated charcoal, intake of large amounts of fluid; supportive and symptomatic therapy. There is no specific antidote. Hemodialysis is ineffective. In case of overdose, you should consult a doctor.
Storage conditions
Storage conditions
Store in a place protected from light at a temperature not exceeding 25 °C.
Keep out of the reach of children.
Shelf life
Shelf life
2 years.
Do not use after expiration date.
Manufacturer
Manufacturer
Vertex, Russia
Additional information
| Shelf life | 2 years. Do not use after the expiration date. |
|---|---|
| Conditions of storage | Store in a light-protected place at a temperature not exceeding 25 °С. Keep out of reach of children. |
| Manufacturer | Vertex, Russia |
| Medication form | nasal spray |
| Brand | Vertex |
Related products
Buy Freenosol, spray 15 ml with delivery to USA, UK, Europe and over 120 other countries.