No products in the cart.
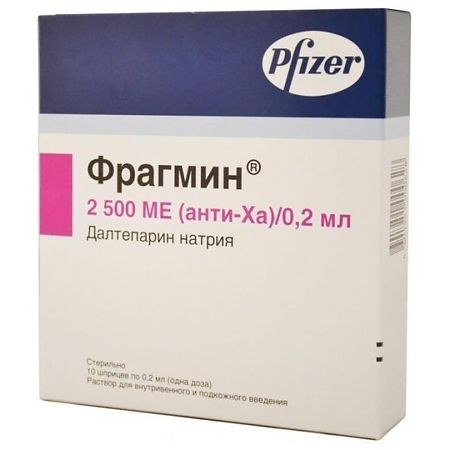
Fragmin, 2500 anti-xa me/0.2 ml 0.2 ml syringes 10 pcs.
€92.07 €76.72
Description
Fragmin has anticoagulant effect.
The anticoagulant effect is primarily due to inhibition of factor Xa, it has little effect on the clotting time.
Fragmin has little effect on platelet adhesion, so has little effect on primary hemostasis.
Pharmacokinetics
The bioavailability after subcutaneous administration is approximately 90%; pharmacokinetic parameters are independent of dose.
The T1/2 after intravenous administration of the drug is 2 hours; after subcutaneous administration, it is 3-5 hours.
In patients with uremia the T1/2 of the drug is prolonged. It is excreted mainly by the kidneys.
Indications
Indications
Acute deep vein thrombosis,
pulmonary embolism,
unstable angina and myocardial infarction (without Q wave on ECG);
prevention of blood coagulation in the extracorporeal circulatory system during hemodialysis and hemofiltration (in patients with acute and chronic renal failure),
prevention of thrombus formation during surgical (including orthopedic) interventions.
Pharmacological effect
Pharmacological effect
Fragmin has an anticoagulant effect.
The anticoagulant effect is primarily due to the inhibition of factor Xa; it has little effect on blood clotting time.
Fragmin has little effect on platelet adhesion, i.e. has little effect on primary hemostasis.
Pharmacokinetics
Bioavailability after subcutaneous administration is approximately 90%; pharmacokinetic parameters are independent of dose.
After intravenous administration of the drug, T1/2 is 2 hours, after subcutaneous administration – 3-5 hours.
In patients with uremia, T1/2 of the drug increases. It is excreted mainly by the kidneys.
Special instructions
Special instructions
Caution should be exercised when prescribing Fragmin to patients with an increased risk of bleeding; This group includes patients with thrombocytopenia, platelet dysfunction, severe liver or kidney failure, uncontrolled hypertension, hypertensive or diabetic retinopathy.
Information on the effectiveness and safety of Fragmin in pediatrics is limited. If such use is necessary, anti-Xa levels should be monitored.
When receiving epidural or spinal anesthesia or performing a spinal tap in patients who are receiving anticoagulant therapy, or who are scheduled to undergo anticoagulant therapy using low molecular weight heparins or heparinoids to prevent thromboembolic complications, there is an increased risk of developing an epidural or spinal hematoma, which in turn can lead to prolonged or permanent paralysis. The risk of such complications increases with the use of indwelling epidural catheters for the administration of analgesics or with the simultaneous use of drugs that affect hemostasis, such as NSAIDs, platelet function inhibitors and other anticoagulants. The risk also increases with trauma and with repeated epidural or lumbar punctures. In such cases, patients should be under constant observation for timely detection of pathological neurological symptoms. If a neurological pathology is detected, emergency intervention (spinal cord decompression) is indicated.
There are no clinical data on the use of Fragmin in patients with pulmonary embolism who also had circulatory disorders, arterial hypotension or shock.
Particular attention is required for patients who, when treated with Fragmin, experience rapid development of thrombocytopenia, or thrombocytopenia with a platelet count of less than 100,000/μl. In such cases, it is recommended to perform an in vitro test for antiplatelet antibodies in the presence of heparin or low molecular weight heparins. If the result of this test is positive or equivocal, or no testing has been done at all, then treatment with Fragmin should be discontinued.
Monitoring the anticoagulant activity of Fragmin is usually not necessary, but it may be necessary when treating special groups of patients: children, patients with underweight or obesity, pregnant women, and patients with an increased risk of bleeding or recurrent thrombosis. Blood samples for analysis of Fragmin activity should be taken during the period when the maximum concentration of the drug in the blood plasma is reached (3-4 hours after subcutaneous injection).
To determine anti-Xa activity, laboratory tests that use a chromogenic substrate are recognized as the method of choice. Activated partial thromboplastin time (aPTT) and thrombin time tests should not be used in this case because these tests are relatively insensitive to the activity of dalteparin sodium. Increasing the dose of Fragmin in order to increase the aPTT may lead to bleeding.
The units of action of Fragmin, unfractionated heparin and other low molecular weight heparins are not equivalent, therefore, when replacing one drug with another, a dose adjustment is required. When using multi-dose vials, unused solution must be destroyed 14 days after the first piercing of the stopper with a needle.
Active ingredient
Active ingredient
Dalteparin sodium
Composition
Composition
0.2 ml solution for injection contains:
active ingredient:
dalteparin sodium 2500 IU,
excipients:
water for injections;
sodium chloride;
sodium hydroxide or hydrochloric acid q.s.
Pregnancy
Pregnancy
Fragmin may be used during pregnancy if the expected effect of therapy exceeds the potential risk to the fetus.
It has not been established whether Fragmin is excreted into breast milk.
Contraindications
Contraindications
Hypersensitivity to dalteparin sodium (including other low molecular weight heparins and heparin),
immune thrombocytopenia (caused by a history of heparin or suspected presence),
bleeding (clinically significant, for example from the gastrointestinal tract against the background of gastric or duodenal ulcer, intracranial bleeding),
pronounced hypocoagulation,
disorders of the blood coagulation system,
septic endocarditis,
recent injuries or surgical interventions on the central nervous system, organs of vision and hearing;
planned spinal or epidural anesthesia or other procedures accompanied by lumbar puncture (this applies to high doses of Fragmin).
Side Effects
Side Effects
Side effects are observed on average in 1% of patients.
From the hematopoietic system and blood coagulation system: bleeding, hematoma at the injection site, reversible non-immune thrombocytopenia, bleeding; in some cases – immune thrombocytopenia (with or without thrombotic complications); development of spinal or epidural hematoma, peritoneal and intracranial bleeding, some of which are fatal.
From the digestive system: transient increase in the activity of liver transaminases (AST, ALT).
Local reactions: pain at the injection site; in some cases – skin necrosis.
Other: allergic reactions, in some cases – anaphylactic reactions.
Interaction
Interaction
When used simultaneously with drugs that affect hemostasis, such as thrombolytic agents, other anticoagulants, NSAIDs, and platelet function inhibitors, the anticoagulant effect of Fragmin may be enhanced; combined use with antihistamines, cardiac glycosides, tetracyclines, and ascorbic acid weakens the effect of dalteparin.
Compatible with intravenous solutions. Fragmin is compatible with isotonic sodium chloride solution (9 mg/ml) and isotonic dextrose solution (50 mg/ml).
Overdose
Overdose
Symptoms: bleeding.
Treatment: administration of protamine (1 mg inhibits 100 IU of dalteparin).
Storage conditions
Storage conditions
Store out of the reach of children at a temperature not exceeding 30°C.
Shelf life
Shelf life
3 years.
Manufacturer
Manufacturer
Pfizer Manufacturing Belgium NV, Belgium
Additional information
| Shelf life | 3 years. |
|---|---|
| Conditions of storage | Keep out of reach of children at temperatures under 30 ° C. |
| Manufacturer | Pfizer MFG. Belgium N.V., Belgium |
| Medication form | solution |
| Brand | Pfizer MFG. Belgium N.V. |
Related products
Buy Fragmin, 2500 anti-xa me/0.2 ml 0.2 ml syringes 10 pcs. with delivery to USA, UK, Europe and over 120 other countries.