No products in the cart.
Fezam, capsules 60 pcs
€14.41 €12.57
Description
Fezam is a combination drug that improves cerebral blood flow and brain metabolism.
Piracetam is a nootropic drug. It has a positive effect on metabolic processes and cerebral circulation. Increases glucose utilization, improves metabolic processes, improves microcirculation in ischemic areas, and inhibits activated platelet aggregation. It has a protective effect in brain damage caused by hypoxia.
Cinnarizine is a calcium channel blocker with a pronounced effect on cerebral vessels. It improves cerebral circulation, increases tissue resistance to hypoxia. It reduces the excitability of the vestibular system.
The two components mutually potentiate each other. With regard to the effect of the drug on the CNS, the sedative effect of cinnarizine prevails. The toxicity of the combination does not exceed the toxicity of the individual components.
The therapeutic effect occurs after 1-6 hours.
Pharmacokinetics
Absorption
Quickly and completely absorbed from the gastrointestinal tract.
Cmax cinnarizine in plasma is noted after 1-4 hours, piracetam – after 2-6 hours.
Distribution
High concentration of cinnarizine after 1-4 hours is noted in the liver, kidneys, heart, lungs, spleen and brain. Cinnarizine binds 91% to plasma proteins.
Piracetam is rapidly distributed to all vital organs. It crosses the blood-brain barrier and is concentrated mainly in the gray matter of the cerebral cortex and cerebellum and also in the nucleus caudatus (caudate nucleus), hippocampus (hippocampus), corpus medianum, geniculus lateralis and plexus choroidalis (choroidal plexus).
Elimination
Piracetam is excreted unchanged in the urine in about 30 hours. 60% of cinnarizine is excreted unchanged in the feces, the rest is excreted with the urine as metabolites in about 5 hours.
Indications
Indications
• Cerebrovascular insufficiency (cerebral atherosclerosis, recovery period of ischemic and hemorrhagic strokes, traumatic brain injuries, encephalopathy of various origins).
• Intoxication.
• Diseases of the central nervous system, accompanied by a decrease in intellectual and mnestic functions (impaired memory, attention, mood).
• Conditions after a traumatic brain injury.
• Psychoorganic syndrome with a predominance of signs of asthenia and adynamia.
• Asthenic syndrome of psychogenic origin.
• Labyrinthopathies – dizziness, tinnitus, nausea, vomiting; nystagmus.
• Meniere’s syndrome.
• Prevention of migraine and kinetosis.
• As part of complex therapy for learning disabilities in children with psychoorganic syndrome.
Pharmacological effect
Pharmacological effect
Pharmacotherapeutic group: Nootropic drug
ATX code: N06BХ
Pharmacological action
Special instructions
Special instructions
Precautions for use
The drug should be prescribed with caution to persons with liver and/or kidney disease. In cases of mild to moderate renal failure (especially if creatinine clearance is less than 60 ml/min), the therapeutic dose should be reduced or the intervals between doses should be increased.
In persons with impaired liver function, monitoring of liver enzyme levels is necessary.
You should avoid drinking alcohol during treatment.
The drug may cause a false-positive reaction when monitoring doping agents in athletes.
The drug increases the activity of thyroid hormones and may cause tremors and anxiety.
Impact on the ability to drive a car and other mechanisms
During treatment, care should be taken when driving vehicles and working with machinery and equipment, since at the beginning of treatment, cinnarizine may cause drowsiness.
Active ingredient
Active ingredient
Piracetam, Cinnarizine
Composition
Composition
1 capsule contains:
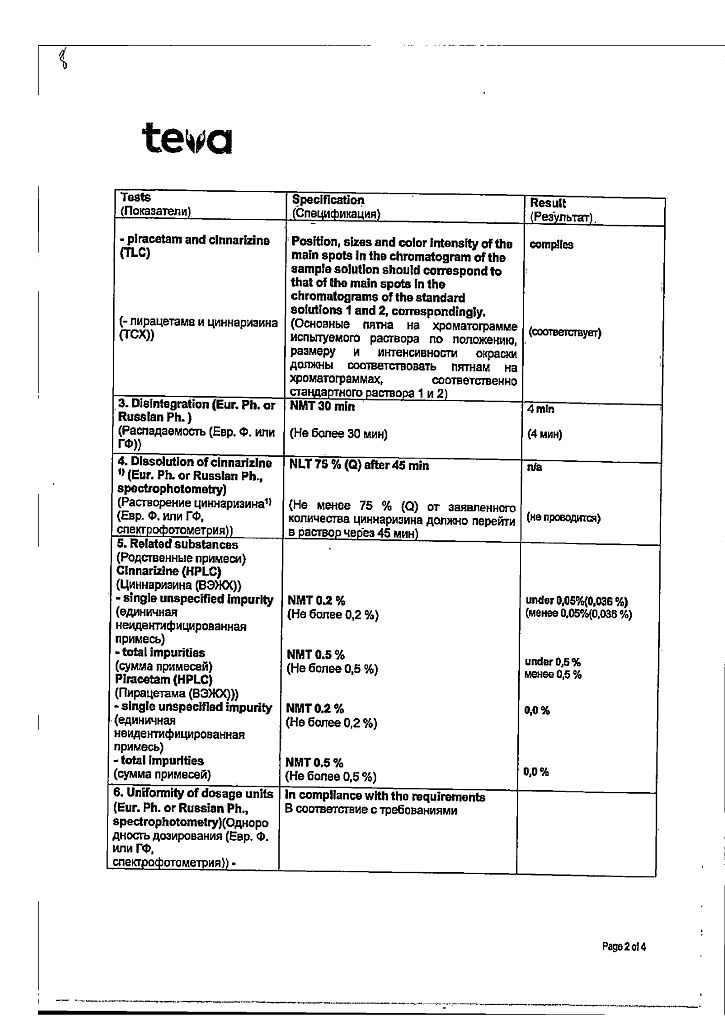
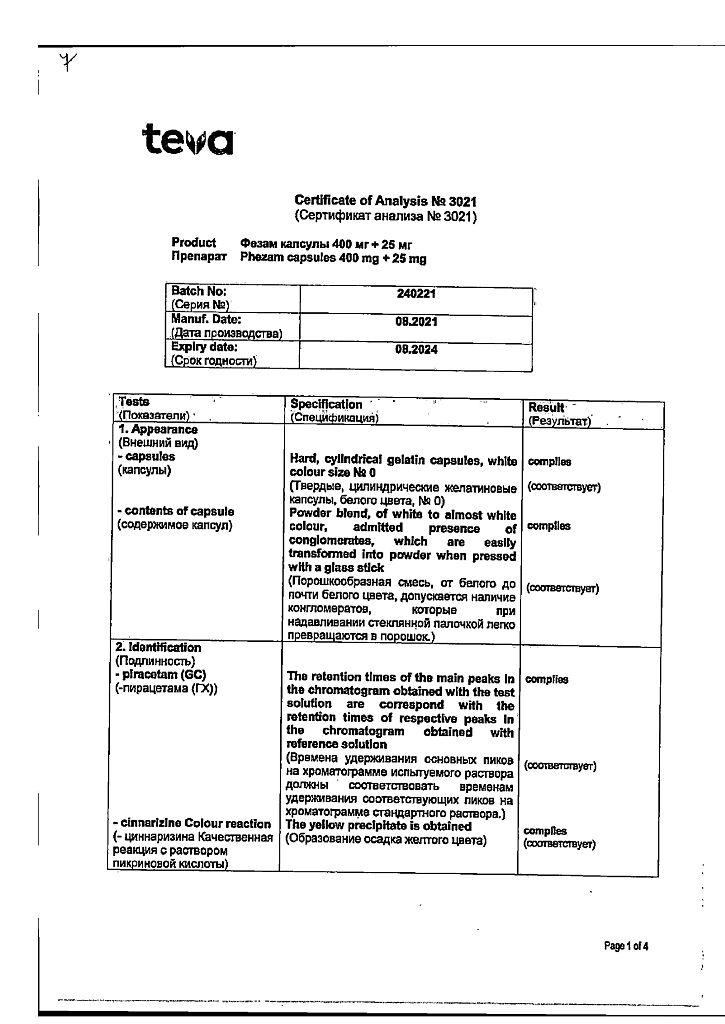
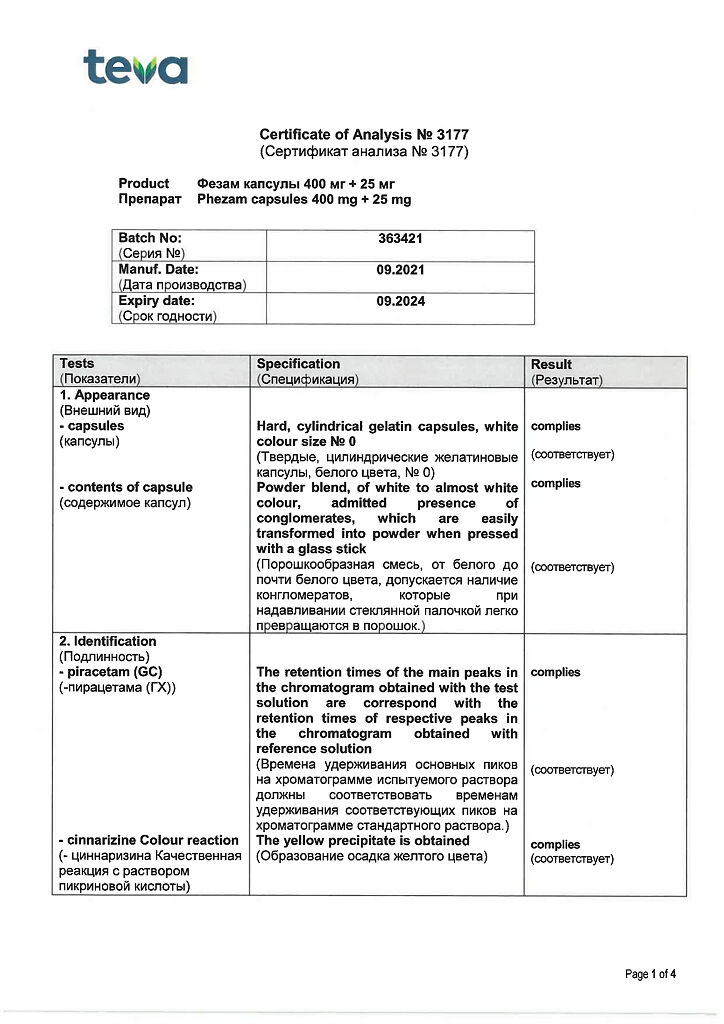
active ingredients – piracetam 400.0 mg, cinnarizine 25.0 mg;
excipients – lactose monohydrate 55.0 mg, colloidal silicon dioxide 15.0 mg, magnesium stearate 5.0 mg. The capsule shell contains titanium dioxide 2%, gelatin 98%.
Pregnancy
Pregnancy
Despite the lack of data on the presence of teratogenic effects of piracetam and cinnarizine, the use of the drug during pregnancy is not recommended. The use of Phezam for pregnant women is indicated only if the expected benefit to the mother outweighs the potential risk to the fetus.
Piracetam is excreted in breast milk, so it is recommended to stop breastfeeding while taking it.
Contraindications
Contraindications
• Hypersensitivity to piracetam, cinnarizine or to any of the excipients included in the drug;
• Severe renal (creatinine clearance < 20 ml/min) and/or liver failure;
• Psychomotor agitation at the time of drug administration;
• Huntington’s chorea;
• Children under 5 years of age;
• Pregnancy and lactation;
• Hemorrhagic stroke;
• Patients with rare hereditary diseases: galactose intolerance, lactase deficiency or glucose-galactose malabsorption (contains lactose monohydrate).
With caution
Parkinson’s disease; conditions associated with increased intraocular pressure; impaired liver and/or kidney function, impaired hemostasis, severe bleeding.
Side Effects
Side Effects
In very rare cases, hypersensitivity reactions – allergic reactions in the form of skin rash, dermatitis, itching, swelling, photosensitivity.
From the gastrointestinal tract: in some cases, increased salivation, nausea, vomiting, diarrhea, and abdominal pain are possible.
From the central and peripheral nervous system: hyperkinesia, nervousness, drowsiness, depression; in isolated cases – dizziness, headaches, ataxia, imbalance, insomnia, confusion, agitation, anxiety, hallucinations.
Other: increased sexual activity.
With long-term therapy, tremor may occur in elderly patients.
Interaction
Interaction
When taken simultaneously with drugs that depress the central nervous system, tricyclic antidepressants and alcohol, the sedative effects are enhanced.
The drug potentiates the effect of nootropic and antihypertensive drugs.
Vasodilators enhance the effect of the drug.
Improves tolerability of antipsychotic drugs and tricyclic antidepressants.
The effect of oral anticoagulants may be enhanced.
Overdose
Overdose
Phezam® is very well tolerated by patients; in case of overdose, no serious side effects requiring discontinuation of the drug are observed.
In case of overdose in children, symptoms of excitement dominate: insomnia, anxiety, euphoria, irritability, tremors and, in rare cases, nightmares, hallucinations and convulsions.
Treatment is symptomatic, which may include hemodialysis. You should perform gastric lavage and induce vomiting. There is no specific antidote.
Storage conditions
Storage conditions
At a temperature not higher than 25 ºС.
Keep out of the reach of children!
Shelf life
Shelf life
3 years. Do not use after the expiration date stated on the package.
Manufacturer
Manufacturer
Balkanpharma – Dupnitsa AD, Bulgaria
Additional information
| Shelf life | 2 years. |
|---|---|
| Conditions of storage | The drug should be stored in a dry place at a temperature of 15 ° to 25 ° C. |
| Manufacturer | Balkanfarma – Dupnitsa AD, Bulgaria |
| Medication form | capsules |
| Brand | Balkanfarma – Dupnitsa AD |
Related products
Buy Fezam, capsules 60 pcs with delivery to USA, UK, Europe and over 120 other countries.