No products in the cart.
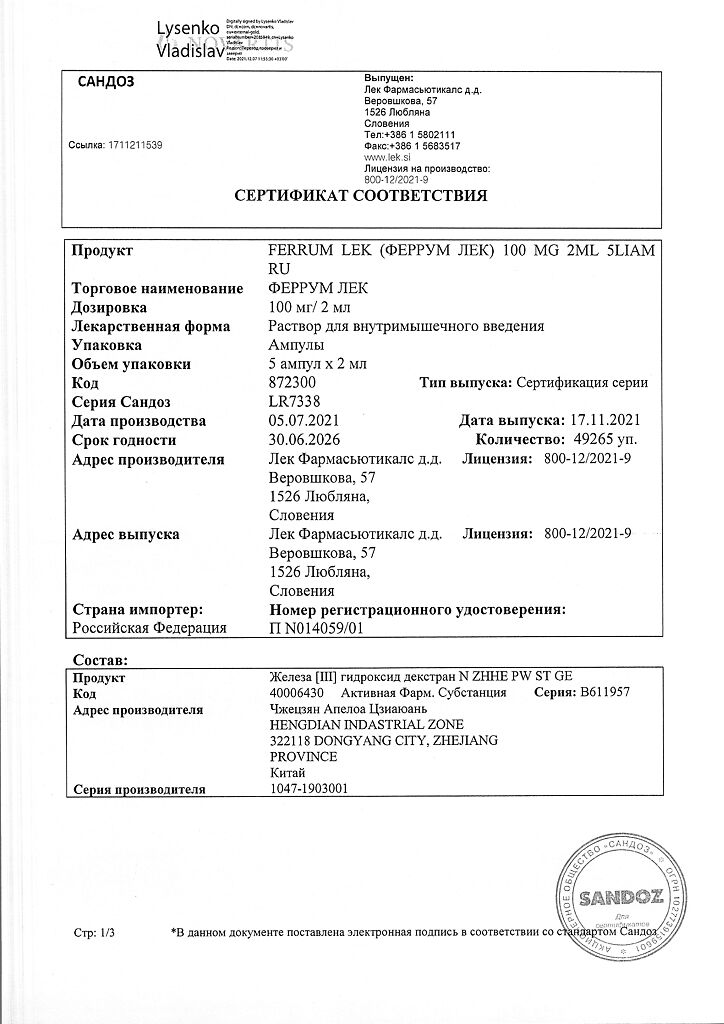
Ferrum Lek, 50 mg/ml 2 ml 5 pcs
€51.57 €42.97
Description
Ferrum Lek is an anti-anemic drug.
Pharmacodynamics
The drug contains trivalent iron in the form of iron trivalent hydroxide complex with dextran. The iron contained in the preparation quickly makes up for the lack of this element in the body (especially in iron-deficiency anemia), restores the content of hemoglobin. Gradual reduction of both clinical symptoms (weakness, fatigue, dizziness, tachycardia, soreness and dryness of the skin) and laboratory indices of iron deficiency is observed during treatment with the preparation.
Pharmacokinetics
After intramuscular administration of the drug, iron rapidly enters the bloodstream: 15% of the dose after 15 minutes, 44% after 30 minutes. The biological T< sub>1/2 is 3-4 days. Iron in complex with transferrin is transported to body cells, where it is used for the synthesis of hemoglobin, myoglobin and some enzymes. The complex of iron (III) hydroxide with dextran is large enough and therefore is not excreted through the kidneys.
Indications
Indications
Treatment of all forms of iron deficiency conditions that require rapid iron replenishment, including the following:
severe iron deficiency due to blood loss;
impaired absorption of iron in the intestines;
conditions for which treatment with oral iron preparations is ineffective or impracticable.
Pharmacological effect
Pharmacological effect
Ferrum Lek is an antianemic drug.
Pharmacodynamics
The drug contains ferric iron in the form of a complex of ferric hydroxide with dextran. Iron, which is part of the drug, quickly replenishes the lack of this element in the body (in particular, in case of iron deficiency anemia), restores hemoglobin content. When treated with the drug, there is a gradual decrease in both clinical symptoms (weakness, fatigue, dizziness, tachycardia, soreness and dry skin) and laboratory indicators of iron deficiency.
Pharmacokinetics
After intramuscular administration of the drug, iron quickly enters the bloodstream: 15% of the dose after 15 minutes, 44% after 30 minutes. Biological T1/2 is 3–4 days. Iron, in combination with transferrin, is transported to the cells of the body, where it is used for the synthesis of hemoglobin, myoglobin and some enzymes. The complex of iron (III) hydroxide with dextran is large enough and therefore is not excreted through the kidneys.
Special instructions
Special instructions
Use only in hospital settings.
When prescribing Ferrum Lek, laboratory tests are required: a general clinical blood test and determination of serum ferritin; it is necessary to exclude impaired iron absorption.
Ferrum Lek is intended for intramuscular administration only.
It is necessary to insert deeply into the gluteal muscle (a needle 5–6 cm long), as well as shifting the tissue when inserting the needle and squeezing the tissue after removing the needle; injected in turn into the right and left gluteal muscles.
An opened ampoule must be used immediately.
The contents of Ferrum Lekne ampoules should be mixed with other drugs.
Treatment with oral forms of iron-containing drugs should begin no earlier than 5 days after the last injection of Ferrum Lek.
If the drug is stored incorrectly, sediment may form; the use of such ampoules is unacceptable.
Active ingredient
Active ingredient
Iron III hydroxide dextran
Composition
Composition
1 ampoule with solution for intramuscular administration contains:
Active ingredient:
iron (III) in the form of iron (III) hydroxide complex with dextran 100 mg;
Excipients:
water for injections.
Pregnancy
Pregnancy
The drug is contraindicated in the first trimester of pregnancy. In the second and third trimesters and during breastfeeding, the use of the drug is possible only if the expected benefit to the mother outweighs the potential harm to the fetus or infant.
Contraindications
Contraindications
hypersensitivity to the components of the drug;
excess iron in the body (hemochromatosis, hemosiderosis);
violation of iron utilization mechanisms (lead anemia, sideroachrestic anemia, thalassemia);
anemia not associated with iron deficiency (for example, hemolytic, megaloblastic, caused by a lack of cyanocobalamin).
I trimester of pregnancy;
Osler-Rendu-Weber syndrome;
infectious kidney diseases in the acute stage;
uncontrolled hyperparathyroidism;
decompensated cirrhosis of the liver;
infectious hepatitis.
With caution: children’s age (up to 4 months).
Side Effects
Side Effects
Arterial hypotension,
joint pain,
enlarged lymph nodes,
increase in body temperature,
headache,
dizziness,
malaise,
dyspepsia (nausea, vomiting);
extremely rarely – allergic or anaphylactic reactions.
Incorrect injection technique can lead to skin staining, pain and inflammation at the injection site.
Interaction
Interaction
Should not be used concomitantly with oral iron-containing medications.
Concomitant use of ACE inhibitors may increase the systemic effects of parenteral iron preparations.
Overdose
Overdose
Symptoms from the drug: iron overdose can lead to acute iron overload and hemosiderosis.
Storage conditions
Storage conditions
The drug should be stored out of the reach of children at a temperature not exceeding 25°C. Do not freeze.
Shelf life
Shelf life
5 years
Manufacturer
Manufacturer
Lek d.d., Slovenia
Additional information
| Shelf life | 5 years |
|---|---|
| Conditions of storage | The drug should be kept out of reach of children at a temperature not exceeding 25 ° C. Do not freeze. |
| Manufacturer | Lek d.d., Slovenia |
| Medication form | solution |
| Brand | Lek d.d. |
Related products
Buy Ferrum Lek, 50 mg/ml 2 ml 5 pcs with delivery to USA, UK, Europe and over 120 other countries.