No products in the cart.
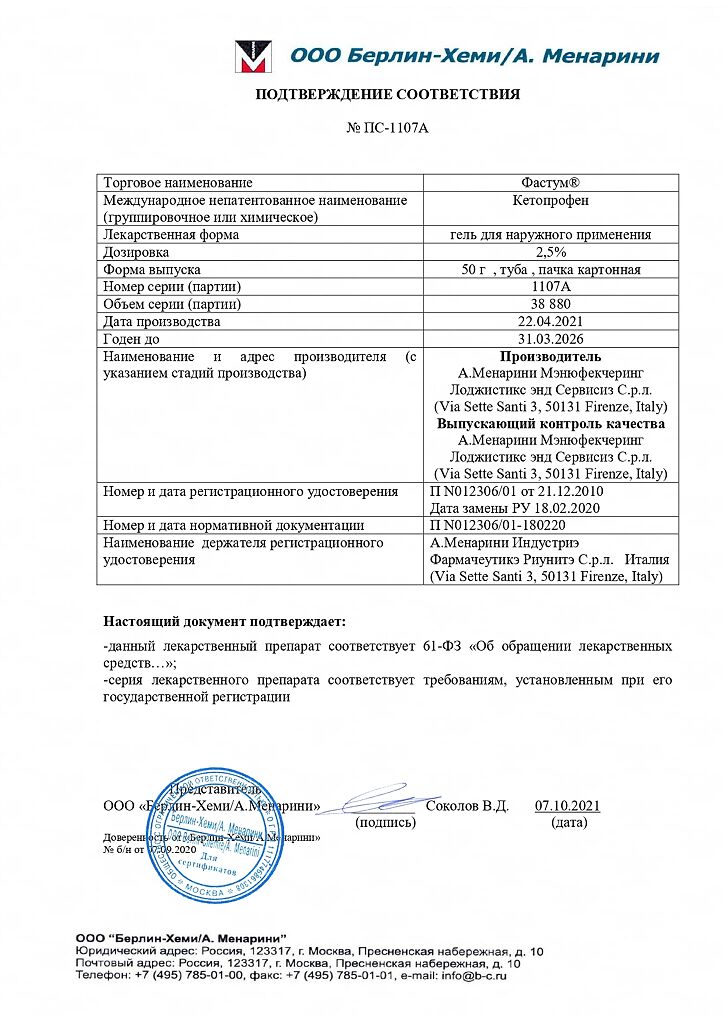
Fastum, gel 2.5% 50 g
€18.10 €15.08
Description
Pharmacotherapeutic group: nonsteroidal anti-inflammatory drugs (NSAIDs) for topical use
ATC code: M02AA10
Pharmacological properties
Pharmacodynamics
The mechanism of action of the drug is associated with inhibition of prostaglandin synthesis. Ketoprofen has analgesic and anti-inflammatory effects. Ketoprofen penetrates through the skin and reaches the site of inflammation, allowing local treatment of painful lesions (joints, tendons, ligaments and muscles).
Pharmacokinetics
When applied topically ketoprofen penetrates into the center of inflammation through the skin, absorption of ketoprofen from the center of inflammation is extremely slow (gel bioavailability – about 5%). After application of ketoprofen in dose of 50-150 mg the concentration in plasma is 0.08-0.15 mkg/ml in 5-8 hours. It practically does not cumulate in the body. The elimination half-life is 1-3 hours.
The binding to plasma proteins is 60-90%. It is eliminated mainly through the kidneys as glucuronide; approximately 90% of the administered dose is eliminated within 24 hours.
Indications
Indications
Symptomatic therapy – reduction of pain and inflammation at the time of use, does not affect the progression of the disease – in the following conditions:
• reactive arthritis (Reiter’s syndrome);
• osteoarthritis of various localizations;
• periarthritis, tendonitis, bursitis, myalgia, neuralgia, radiculitis;
• injuries of the musculoskeletal system (including sports), bruises of muscles and ligaments, sprains, ruptures of ligaments and muscle tendons.
Pharmacological effect
Pharmacological effect
Pharmacotherapeutic group: nonsteroidal anti-inflammatory drugs (NSAIDs) for topical use
ATX code: M02AA10
Pharmacological properties
Pharmacodynamics
Ketoprofen is one of the most effective cyclooxygenase inhibitors. It also inhibits lipoxygenase and bradykinin activity. Stabilizes lysosomal membranes and prevents the release of enzymes involved in the inflammatory process. The main properties of ketoprofen are analgesic, anti-inflammatory and anti-edema effects. Ketoprofen does not have a negative effect on the condition of articular cartilage.
Pharmacokinetics
Suction
Ketoprofen, when applied topically in the form of a gel, does not accumulate in the body.
The bioavailability of the gel is about 5%. The maximum concentration of ketoprofen in the blood plasma is achieved 6 hours after application of the drug.
Distribution
Penetrates into joint tissues, incl. into the synovial fluid, and reaches therapeutic concentrations there. The concentration of the drug in the blood plasma is extremely low.
Metabolism and excretion
Ketoprofen is metabolized in the liver to form conjugates, which are excreted primarily by the kidneys. The metabolism of ketoprofen does not depend on age, the presence of severe renal failure or liver cirrhosis. Excretion of ketoprofen by the kidneys is slow.
Special instructions
Special instructions
Do not apply to open wounds or inflamed skin!
It is necessary to avoid getting the gel in the eyes, on the skin around the eyes, mucous membranes.
If skin reactions occur, including those that develop during combined use with octocrylene-containing drugs, treatment should be stopped immediately.
Patients suffering from bronchial asthma in combination with chronic rhinitis, chronic sinusitis and/or polyposis of the nose or paranasal sinuses have a higher risk of developing allergic reactions when using aspirin and/or NSAIDs than the rest of the population.
To reduce the risk of developing photosensitivity, it is recommended to protect the skin areas treated with the gel with clothing from exposure to UV radiation throughout the entire treatment period and for another 2 weeks after stopping use.
The recommended duration of treatment should not be exceeded due to the increased risk of contact dermatitis and photosensitivity reactions over time.
You should wash your hands thoroughly after each application of the drug.
Impact on the ability to drive vehicles and machinery
There is no data on the negative impact of the drug Fastum® on the ability to drive vehicles and engage in other potentially hazardous activities that require concentration and speed of psychomotor reactions.
Active ingredient
Active ingredient
Ketoprofen
Composition
Composition
100 g of gel contains:
Active ingredient: ketoprofen – 2.50 g.
Excipients: carbomer, ethanol 96%, neroli flavor, lavandin flavor, trolamine (triethanolamine), purified water.
Pregnancy
Pregnancy
Pregnancy
Use up to 20 weeks of pregnancy
Since the safety of ketoprofen in pregnant women has not been assessed, the use of ketoprofen should be avoided before 20 weeks of pregnancy.
Use after 20 weeks of pregnancy
The drug Fastum® is contraindicated in more than 20 weeks of pregnancy. NSAIDs should not be used by women from the 20th week of pregnancy due to the possible development of oligohydramnios and/or kidney pathology in newborns (neonatal renal dysfunction).
During the third trimester of pregnancy, all prostaglandin synthetase inhibitors, including ketoprofen, can have toxic effects on the heart, lungs and kidneys of the fetus.
At the end of pregnancy, bleeding time for mother and baby may increase.
NSAIDs may delay the onset of labor.
Breast-feeding
To date, there is no data on the penetration of ketoprofen into breast milk, therefore the use of Fastum® during breastfeeding is not recommended.
Contraindications
Contraindications
• Hypersensitivity to ketoprofen or any of the excipients of the drug, as well as to salicylates, tiaprofenic acid or other NSAIDs, fenofibrate; history of skin allergies to sunscreens and perfumes;
• complete or incomplete combination of bronchial asthma, recurrent polyposis of the nose and paranasal sinuses and intolerance to acetylsalicylic acid or other NSAIDs (including a history);
• pregnancy over 20 weeks;
• children’s age (up to 15 years);
• violation of the integrity of the skin in the area where the gel is applied (eczema, acne, weeping dermatitis, open or infected wound);
• history of photosensitivity reactions;
• exposure to sunlight, incl. indirect sunlight and UV irradiation in a solarium throughout the entire treatment period and for another 2 weeks after stopping treatment with the drug.
With caution
You should consult your doctor before using Fastum® if you:
• impaired liver and/or kidney function;
• erosive and ulcerative lesions of the gastrointestinal tract;
• blood diseases;
• bronchial asthma;
• chronic heart failure;
• hepatic porphyria (exacerbation);
• pregnancy up to 20 weeks.
Side Effects
Side Effects
According to the World Health Organization (WHO), adverse reactions are classified according to their frequency as follows: very common (≥ 1/10), common (≥ 1/100 to < 1/10), uncommon (≥ 1/1000 to < 1/100), rare (≥ 1/10,000 to < 1/1000), very rare (< 1/10,000); frequency unknown - based on available data, it was not possible to determine the frequency of occurrence.
Immune system disorders
Frequency unknown: anaphylactic shock, angioedema (Quincke’s edema), hypersensitivity reactions.
Gastrointestinal disorders
Very rare: peptic ulcer, bleeding, diarrhea.
Skin and subcutaneous tissue disorders
Uncommon: local skin reactions such as erythema, eczema, itching and burning;
Rarely: photosensitivity reactions, urticaria.
There have been rare reports of more severe reactions, such as bullous or phlyctenulous eczema, which may spread beyond the site of application or become generalized.
Renal and urinary tract disorders
Very rare: deterioration of renal function in patients with chronic renal failure.
If any side effects occur, you should stop using the drug and consult a doctor.
Interaction
Interaction
When ketoprofen is used externally in a gel dosage form, it is possible to enhance the effect of drugs that cause photosensitivity.
Patients taking coumarin anticoagulants are advised to regularly monitor the international normalized ratio (INR).
Ketoprofen, like other NSAIDs, may reduce the elimination of methotrexate and increase its toxicity.
Interactions with other drugs and the effect on their elimination are not significant.
Overdose
Overdose
Symptoms
Overdose is unlikely when the drug is used externally.
If the drug is ingested, systemic adverse reactions may develop.
Treatment
In case of overdose when using the drug externally, the skin should be thoroughly rinsed under running water.
In case of ingestion of the drug, symptomatic treatment and supportive care are necessary as in case of overdose with oral forms.
Storage conditions
Storage conditions
Store at a temperature not exceeding 30 °C.
Keep the medicine out of the reach of children!
Shelf life
Shelf life
5 years. Do not use after the expiration date indicated on the package!
Manufacturer
Manufacturer
A. Menarini Manufacturing Logistics and Services S, Italy
Additional information
| Shelf life | 5 years. Do not use after the expiration date stated on the package! |
|---|---|
| Conditions of storage | Store at a temperature not higher than 30 ° C. Keep the medicine out of reach of children! |
| Manufacturer | A.Menarini Manufechering Logistics and Services S, Italy |
| Medication form | gel for external use |
| Brand | A.Menarini Manufechering Logistics and Services S |
Other forms…
Related products
Buy Fastum, gel 2.5% 50 g with delivery to USA, UK, Europe and over 120 other countries.