No products in the cart.
Exchol, 500 mg 100 pcs
€1.00
Out of stock
(E-mail when Stock is available)
Description
- dissolution of small and medium cholesterol stones with functioning gallbladder;
- biliary reflux gastritis;
- primary biliary cirrhosis in the absence of signs of decompensation (symptomatic treatment);
- Primary sclerosing cholangitis;
- cystic fibrosis (cystic fibrosis);
- non-alcoholic steatohepatitis;
- alcoholic liver disease;
- dyskinesia of the biliary tract.
.
Active ingredient
Active ingredient
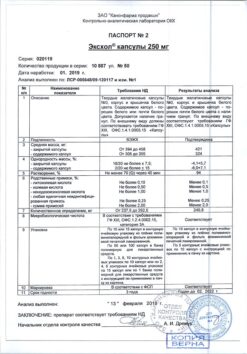
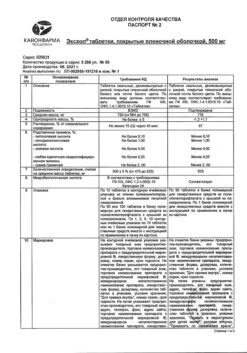
Composition
Composition
1 film-coated tablet contains:
active ingredient: ursodeoxycholic acid 500 mg;
excipients: calcium hydrophosphate dihydrate 40 mg, calcium stearate 7 mg, sodium carboxymethyl starch 28 mg, potato starch 33.5 mg, mannitol 58 mg, macrogol (polyethylene glycol 4000) 3.5 mg, povidon K-30 30 mg;
film coating composition: Opadray white 20 mg, including: hypromellose (hydroxypropyl methylcellulose) 6.75°mg, hyprolose (hydroxypropyl cellulose) 6.75 mg, talc 4 mg, titanium dioxide 2.5 mg.
How to take, the dosage
How to take, the dosage
Ingestion, without chewing, with plenty of water.
Dissolution of cholesterol gallstones
The recommended dose of Exole® is 10 mg/kg body weight per day.
The entire daily dose is taken once at bedtime. The course of treatment Ë 6 – 12 months. To prevent the recurrent formation of stones it is recommended to take the preparation during several months after the dissolution of stones.
Treatment of biliary reflux gastritis
250 mg a day before going to bed. The course of treatment – from 10-14 days to 6 months, if necessary – up to 2 years.
Symptomatic treatment of primary biliary cirrhosis. The daily dose depends on body weight and is 10-15 mg/kg/day (if necessary – up to 20 mg/kg) in 2-3 times during the first 3 months of treatment. After improvement of “hepatic” parameters, the daily dose can be used once in the evening. The duration of treatment course is not limited. In rare cases, in the beginning, clinical symptoms may worsen (itching becomes more frequent). In this case daily dose should be reduced and then the dosage should be gradually increased (increasing daily dose weekly) until the recommended dosage regimen is achieved.
In chronic hepatitis of different genesis, nonalcoholic steatohepatitis, alcoholic liver disease
Exole® is prescribed in a daily dose of 10-15 mg of ursodeoxycholic acid per 1 kg of body weight in 2-3 doses continuously during a long period (6-12 months and more).
In primary sclerosing cholangitis and cystic fibrosis (cystic fibrosis)
In primary sclerosing cholangitis: 12-15 mg/kg/day (up to 20 mg/kg) of body weight during 2-3 intakes per day. Duration of use – from 6 months to several years. In cystic fibrosis (cystic fibrosis): 20-30 mg/kg per day in 2-3 doses. The duration of treatment – from 6 months to several years.
In biliary dyskinesia: The average daily dose is 10 mg/kg in 2 intakes during 2 weeks to 2 months. If necessary the course of treatment may be repeated.
If the dosage regimen cannot be observed it is recommended to use the form of capsules, Exole, 250 mg.
Ursodeoxycholic acid is prescribed individually to children over 3 years old at the rate of 10-20 mg/kg/day.
Interaction
Interaction
Antacids containing aluminum, ion exchange resins (colestyramine, colestipol) reduce absorption of ursodeoxycholic acid. If the use of drugs containing at least one of these substances is still necessary, they should be taken at least 2 hours before taking Exxol.
Ursodeoxycholic acid may enhance absorption of cyclosporine from intestine. Therefore, in patients taking cyclosporine its blood concentration should be monitored and the dose of cyclosporine should be adjusted if necessary.
In individual cases the drug Exxol may reduce absorption of ciprofloxacin.
Hypolipidemic drugs (especially clofibrate), estrogens, neomycin or progestins increase bile cholesterol saturation and may decrease the ability to dissolve cholesterol bile concretions.
Special Instructions
Special Instructions
For successful dissolution of gallstones it is necessary that the stones are purely cholesterol, their size does not exceed 15-20 mm, the gallbladder must remain functional and not be filled with stones by more than half, the biliary tracts must retain their full function.
During the first three months of treatment, the activity of liver transaminases, alkaline phosphatase (ALP), gamma-glutamintransferase (GGT), bilirubin concentration in serum every 4 weeks, and then every 3 months.
Cholistography must be performed every 4 weeks during first 3 months of treatment, further – every 3 months. The control of treatment efficacy is to be performed every 6 months by ultrasound and X-ray examination of biliary tract during the first year of therapy.
The detection of invisible gallbladder during the treatment is a sign that the complete dissolution of concrements has not occurred, and the treatment must be stopped. In cases of calcinosis of stones, weak gallbladder contractility or frequent attacks of colic, Exxol® should not be used. If within 6-12 months after the beginning of treatment partial dissolution of calculi has not occurred, it is unlikely that the treatment will be effective. After complete dissolution of concrements it is recommended to continue use for at least 3 months in order to promote dissolution of residual concrements too small for their detection and for prevention of recurrence of stone formation.
In patients with diarrhea the drug dosage should be reduced. In case of persistent diarrhea the treatment should be discontinued.
If prolonged therapy with high doses of ursodeoxycholic acid (up to 30 mg/kg/day) is necessary, the use of the drug may lead to serious adverse events in patients with primary sclerosing cholangitis.
Contraindications
Contraindications
Hypersensitivity to ursodeoxycholic acid or any of the ingredients of the drug, X-ray-positive (high calcium) gallstones, non-functioning gallbladder, acute infectious and inflammatory diseases of the gallbladder, bile ducts and intestines, decompensated liver cirrhosis, marked liver and/or renal failure, pancreatitis, adults and children with body weight less than 34 kg, children under 3 years for this LF.
Side effects
Side effects
WHO (World Health Organization) Classification of the frequency of side effects:
often – from â¥1/100 to < 1/10 appointments (>1% and < 10%);
infrequent – from â¥1/1000 to < 1/100 appointments (>01% and < 1%);
rare – â¥1/10000 to < 1/1000 appointments (>0.01% and < 0.1%);
very rare – < 1/10000 appointments (< 0.01%).
Gastrointestinal tract: frequently – unformed stools, diarrhea (may be dose-dependent); very rarely – during treatment of primary biliary cirrhosis transient decompensation of liver cirrhosis may be observed (passes after drug withdrawal).
Liver and biliary tract disorders: very rarely – calcification of gallstones, in treatment of advanced stages of primary biliary cirrhosis – decompensation of liver cirrhosis, which disappears after discontinuation of the medicine.
Skin disorders: very rare – allergic reactions (including urticaria).
Overdose
Overdose
No cases of overdose were found. In case of overdose symptomatic treatment is carried out.
Similarities
Similarities
Additional information
| Weight | 0.121 kg |
|---|---|
| Shelf life | 2 years |
| Conditions of storage | In a light-protected place at a temperature not exceeding 25 °C. |
| Manufacturer | Kanonfarma Production ZAO, Russia |
| Medication form | pills |
| Brand | Kanonfarma Production ZAO |
Other forms…
Related products
Buy Exchol, 500 mg 100 pcs with delivery to USA, UK, Europe and over 120 other countries.