No products in the cart.
Eufillin, 24 mg/ml 5 ml 10 pcs
€2.16 €1.96
Description
Euphylline is a bronchodilator and an FDE inhibitor. It is ethylenediamine salt of theophylline (which facilitates solubility and increases absorption). It has a bronchodilator effect, apparently due to a direct relaxing effect on the smooth muscles of the airways and blood vessels of the lungs. It is believed that this effect is caused by selective inhibition of the activity of specific FDEs, which leads to an increase in the intracellular concentration of cAMP. The results of experimental studies in vitro show that the main role seems to be played by type III and type IV isoenzymes. Suppression of the activity of these isoenzymes may also cause some side effects of aminophylline (theophylline), including vomiting, arterial hypotension and tachycardia. It blocks adenosine (purine) receptors, which may be a factor of action on the bronchi.
It reduces airway hyperresponsiveness associated with the late phase of the response caused by inhaling allergens through an unknown mechanism that is not related to FDE inhibition or adenosine blockade. There are reports that aminophylline increases the number and activity of T suppressors in peripheral blood.
Increases mucociliary clearance, stimulates diaphragm contraction, improves respiratory and intercostal muscle function, stimulates the respiratory center, increases its carbon dioxide sensitivity and improves alveolar ventilation, which ultimately leads to a reduction in the severity and frequency of apnea episodes. By normalizing respiratory function, it promotes blood oxygenation and decreases carbon dioxide concentration. It enhances lung ventilation in conditions of hypokalemia.
It has a stimulating effect on heart activity, increases strength and heart rate, increases coronary blood flow and increases myocardial oxygen demand. Reduces the tone of blood vessels (mainly vessels of the brain, skin and kidneys). It has a peripheral venodilator effect, reduces pulmonary vascular resistance, lowers the pressure in the small circle of the circulation. Increases renal blood flow, has a moderate diuretic effect. Dilates extrahepatic biliary tracts. Stabilizes membranes of mast cells, inhibits release of mediators of allergic reactions. Inhibits platelet aggregation (inhibits platelet activation factor and PgE2α), increases resistance of red blood cells to deformation (improves rheological properties of blood), reduces thrombosis and normalizes microcirculation. It has a tocolytic effect and increases the acidity of gastric juice. In high doses it has epileptogenic effect.
Pharmacokinetics
In the body aminophylline is metabolized at physiological pH values with release of free theophylline. Bronchodilator properties are evident at plasma theophylline concentrations of 10-20 µg/ml. Concentrations above 20 mg/ml are toxic. The excitatory effect on the respiratory center is realized at lower concentrations of 5-10 µg/ml.
Theophylline’s binding to plasma proteins is approximately 40%; in newborns as well as in adults with disease, the binding decreases. Binding to plasma proteins is approximately 60% in adults, 36% in newborns, and 36% in cirrhotic patients. It penetrates the placental barrier (the concentration in the fetal serum is slightly higher than in the mother’s serum). It is excreted with the breast milk.
Theophylline is metabolized in the liver with the participation of several cytochrome P450 isoenzymes, the most important of which is CYP1A2. During metabolism 1,3-dimethylureic acid, 1-methylureic acid and 3-methylxanthine are formed. These metabolites are excreted in the urine. In adults, 10% is excreted unchanged. In infants a significant portion is excreted as caffeine (due to immaturity of pathways of its further metabolism), unchanged – 50%.
Significant individual differences of hepatic metabolism rate of theophylline cause marked variability of clearance values, plasma concentrations and elimination half-life. Such factors as age, tobacco smoking habits, diet, diseases, and concomitant drug therapy affect hepatic metabolism.
Theophylline’s T1/2 in non-smoking patients with bronchial asthma almost without pathological changes from other organs and systems is 6-12 hours, in smokers – 4-5 hours, in children – 1-5 hours, in newborns and premature infants – 10-45 hours.
T1/2 of theophylline is increased in elderly persons and in patients with heart failure or liver disease.
Clearance is decreased in heart failure, liver function disorders, chronic alcoholism, pulmonary edema, chronic obstructive pulmonary disease.
Ethylenediamine does not affect the pharmacokinetics of theophylline.
Indications
Indications
status asthmaticus (additional therapy),
apnea of newborns,
cerebrovascular accident of ischemic type (as part of combination therapy),
left ventricular failure with bronchospasm and Cheyne-Stokes type breathing disorder,
edematous syndrome of renal origin (as part of complex therapy);
acute and chronic heart failure (as part of combination therapy).
Pharmacological effect
Pharmacological effect
Eufillin is a bronchodilator, a PDE inhibitor. It is the ethylenediamine salt of theophylline (which facilitates solubility and increases absorption). It has a bronchodilator effect, apparently due to a direct relaxing effect on the smooth muscles of the respiratory tract and blood vessels of the lungs. It is believed that this effect is caused by selective inhibition of the activity of specific PDEs, which leads to an increase in the intracellular concentration of cAMP. The results of in vitro experimental studies show that the main role appears to be played by type III and IV isoenzymes. Suppression of the activity of these isoenzymes may also cause some side effects of aminophylline (theophylline), including. vomiting, arterial hypotension and tachycardia. Blocks adenosine (purine) receptors, which may be one of the factors affecting the bronchi.
Reduces airway hyperresponsiveness associated with the late phase response caused by inhaled allergens through an unknown mechanism that is not due to PDE inhibition or blockade of adenosine. There are reports that aminophylline increases the number and activity of T-suppressor cells in the peripheral blood.
Increases mucociliary clearance, stimulates contraction of the diaphragm, improves the function of the respiratory and intercostal muscles, stimulates the respiratory center, increases its sensitivity to carbon dioxide and improves alveolar ventilation, which ultimately leads to a decrease in the severity and frequency of apnea episodes. By normalizing respiratory function, it helps saturate the blood with oxygen and reduce the concentration of carbon dioxide. Strengthens ventilation of the lungs in conditions of hypokalemia.
It has a stimulating effect on the activity of the heart, increases strength and heart rate, increases coronary blood flow and increases the myocardial oxygen demand. Reduces the tone of blood vessels (mainly those of the brain, skin and kidneys). It has a peripheral venodilating effect, reduces pulmonary vascular resistance, and lowers pressure in the pulmonary circulation. Increases renal blood flow and has a moderate diuretic effect. Expands extrahepatic bile ducts. Stabilizes mast cell membranes, inhibits the release of mediators of allergic reactions. Inhibits platelet aggregation (suppresses platelet activating factor and PgE2α), increases the resistance of red blood cells to deformation (improves the rheological properties of blood), reduces thrombus formation and normalizes microcirculation. It has a tocolytic effect, increases the acidity of gastric juice. In high doses it has an epileptogenic effect.
Pharmacokinetics
In the body, aminophylline is metabolized at physiological pH values to release free theophylline. Bronchodilating properties appear at plasma theophylline concentrations of 10-20 mcg/ml. Concentrations above 20 mg/ml are toxic. The stimulating effect on the respiratory center is realized at a lower concentration – 5-10 mcg/ml.
Theophylline binding to plasma proteins is approximately 40%; in newborns, as well as in adults with diseases, binding decreases. Plasma protein binding in adults is about 60%, in newborns – 36%, in patients with liver cirrhosis – 36%. Penetrates the placental barrier (the concentration in the fetal blood serum is slightly higher than in the maternal serum). Excreted in breast milk.
Theophylline is metabolized in the liver with the participation of several cytochrome P450 isoenzymes, the most important of which is CYP1A2. During metabolism, 1,3-dimethyluric acid, 1-methyluric acid and 3-methylxanthine are formed. These metabolites are excreted in the urine. 10% is excreted unchanged in adults. In newborns, a significant portion is excreted in the form of caffeine (due to the immaturity of the pathways for its further metabolism), unchanged – 50%.
Significant individual differences in the rate of hepatic metabolism of theophylline are the cause of pronounced variability in clearance values, plasma concentrations, and half-life. Hepatic metabolism is influenced by factors such as age, addiction to tobacco smoking, diet, diseases, and concurrent drug therapy.
T1/2 of theophylline in non-smoking patients with bronchial asthma with virtually no pathological changes in other organs and systems is 6-12 hours, in smokers – 4-5 hours, in children – 1-5 hours, in newborns and premature babies – 10-45 hours.
T1/2 of theophylline increases in the elderly and in patients with heart failure or liver disease.
Clearance decreases with heart failure, liver dysfunction, chronic alcoholism, pulmonary edema, chronic obstructive pulmonary disease.
Ethylenediamine does not affect the pharmacokinetics of theophylline.
Special instructions
Special instructions
Use with caution in severe coronary insufficiency (acute phase of myocardial infarction, angina pectoris), widespread atherosclerosis, hypertrophic obstructive cardiomyopathy, frequent ventricular extrasystoles, increased convulsive readiness, liver and/or renal failure, gastric and duodenal ulcers (history), recent bleeding from the gastrointestinal tract, uncontrolled hypothyroidism (possibility of cumulation) or thyrotoxicosis, with prolonged hyperthermia, gastroesophageal reflux, prostate hypertrophy, in elderly patients, in children (especially orally).
Correction of the aminophylline dosage regimen may be required in case of heart failure, liver dysfunction, chronic alcoholism, fever, acute respiratory infections.
In elderly patients, a dose reduction may be required.
When replacing the used dosage form of aminophylline with another, clinical observation and monitoring of the concentration of theophylline in the blood plasma is necessary.
Aminophylline is not used simultaneously with other xanthine derivatives. During the treatment period, you should avoid eating foods containing xanthine derivatives (strong coffee, tea).
Use with caution simultaneously with anticoagulants, other theophylline or purine derivatives.
Concomitant use with beta-blockers should be avoided.
Aminophylline should not be used simultaneously with glucose solution.
Active ingredient
Active ingredient
Aminophylline
Composition
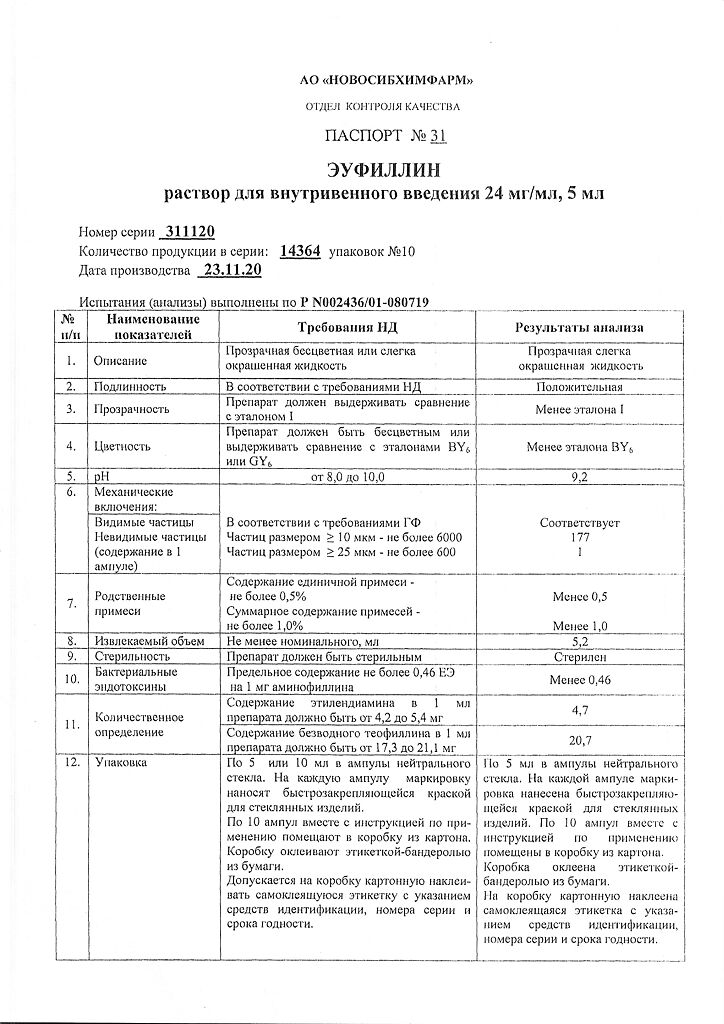
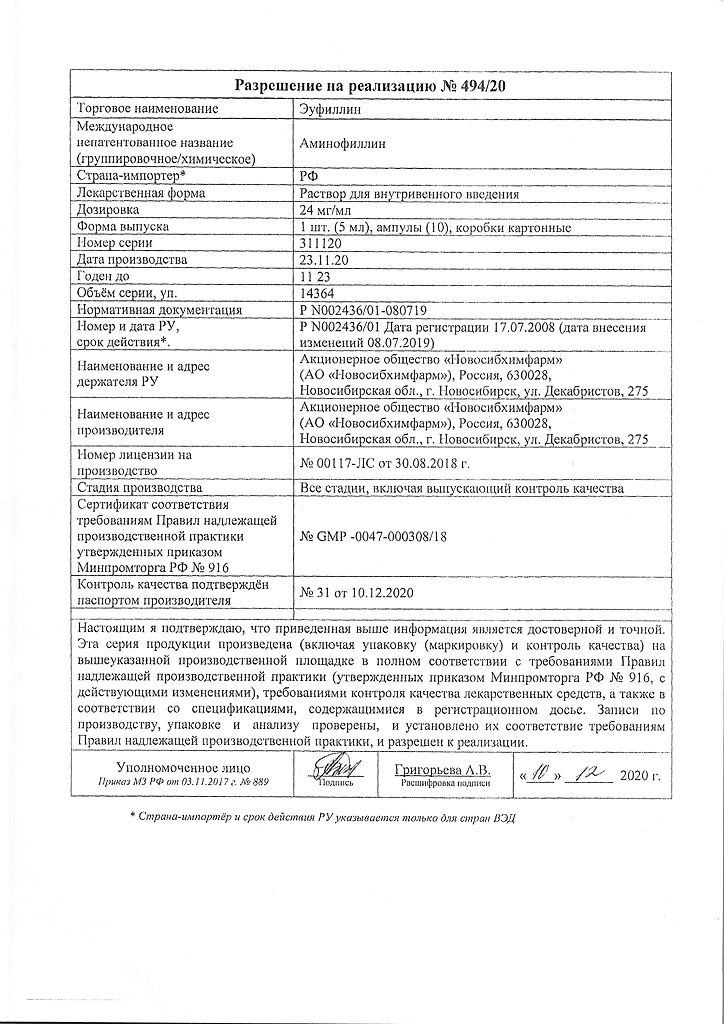
Composition
1 ml of solution for intravenous administration contains:
active substance:
aminophylline 24 mg.
Contraindications
Contraindications
hypersensitivity to aminophylline and theophylline.
severe arterial hyper- or hypotension,
tachyarrhythmias,
peptic ulcer of the stomach and duodenum in the acute phase,
hyperacid gastritis,
severe impairment of liver and/or kidney function,
epilepsy,
hemorrhagic stroke,
hemorrhage in the retina of the eye,
simultaneous use with ephedrine in children,
children’s age (up to 12 years).
Side Effects
Side Effects
From the central nervous system: dizziness, sleep disturbances, anxiety, tremor, convulsions.
From the cardiovascular system: palpitations, heart rhythm disturbances; with rapid intravenous administration – the appearance of pain in the heart, decreased blood pressure, tachycardia (including in the fetus when taken in the third trimester of pregnancy), arrhythmias, decreased blood pressure, cardialgia, increased frequency of angina attacks.
From the digestive system: nausea, vomiting, gastroesophageal reflux, heartburn, exacerbation of peptic ulcer, diarrhea; with prolonged ingestion – anorexia.
From the urinary system: albuminuria, hematuria.
Allergic reactions: skin rash, itching, fever.
Metabolism: rarely – hypoglycemia.
Local reactions: compaction, hyperemia, pain at the injection site; when used rectally, irritation of the rectal mucosa, proctitis.
Other: chest pain, tachypnea, flushing, albuminuria, hematuria, hypoglycemia, increased diuresis, increased sweating.
Interaction
Interaction
When used simultaneously with sympathomimetics, a mutual enhancement of action occurs; with beta-blockers and lithium preparations – the effect is mutually reduced. The intensity of action of aminophylline may decrease (due to an increase in its clearance) when used simultaneously with phenobarbital, rifampicin, isoniazid, carbamazepine, sulfinpyrazone, phenytoin, as well as in smokers.
The intensity of action of aminophylline may increase (due to a decrease in its clearance) when used simultaneously with antibiotics from the macrolide group, lincomycin, with quinolones, allopurinol, beta-blockers, cimetidine, disulfiram, fluvoxamine, hormonal contraceptives for oral administration, isoprenaline, viloxazine and with influenza vaccination.
Xanthine derivatives may potentiate hypokalemia caused by the action of β2-adrenergic receptor stimulants, corticosteroids and diuretics.
Antidiarrheal drugs and enterosorbents reduce the absorption of aminophylline.
Pharmaceutically incompatible with acid solutions.
Overdose
Overdose
In case of overdose, facial skin flushing, insomnia, motor agitation, anxiety, photophobia, anorexia, diarrhea, nausea, vomiting, pain in the epigastric region, gastrointestinal bleeding, tachycardia, ventricular arrhythmias, tremor, generalized convulsions, hyperventilation, and a sharp decrease in blood pressure are observed. In severe poisoning, epileptoid seizures may develop (especially in children without any warning signs), hypoxia, metabolic acidosis, hyperglycemia, hypokalemia, skeletal muscle necrosis, confusion, renal failure with myoglobinuria.
Treatment of overdose depends on the clinical picture and includes drug withdrawal, stimulation of its removal from the body (forced diuresis, hemosorption, plasma sorption, hemodialysis, peritoneal dialysis) and the prescription of symptomatic drugs. Diazepam (by injection) is used to relieve seizures. Barbiturates should not be used. In case of severe intoxication (eufillin content more than 50 g/l), hemodialysis is recommended.
Storage conditions
Storage conditions
In a place protected from light at a temperature of 10 to 25C. Freezing is unacceptable.
Shelf life
Shelf life
3 years. Do not use after the expiration date indicated on the package.
Manufacturer
Manufacturer
Novosibkhimpharm, Russia
Additional information
| Shelf life | 3 years. Do not use after the expiry date stated on the package. |
|---|---|
| Conditions of storage | In a light-protected place at a temperature of 10 to 25C. Freezing is unacceptable. |
| Manufacturer | Novosibkhimpharm, Russia |
| Medication form | solution |
| Brand | Novosibkhimpharm |
Other forms…
Related products
Buy Eufillin, 24 mg/ml 5 ml 10 pcs with delivery to USA, UK, Europe and over 120 other countries.