No products in the cart.
Espiro, 25 mg 30 pcs
€25.61 €21.34
Description
Espiro is a potassium-saving diuretic.
Pharmic action: Eplerenone has relative selectivity for mineralocorticoid receptors in humans compared to glucocorticoid, progesterone, and androgen receptors and blocks their binding to aldosterone, a key hormone of the renin-angiotensin-aldosterone system (RAAS) that is involved in blood pressure regulation and the pathogenesis of cardiovascular disease.
Eplerenone causes a persistent increase in plasma renin and serum aldosterone levels. Subsequently, renin secretion is inhibited by aldosterone through a feedback mechanism. At the same time, increase of renin activity or circulating aldosterone level does not affect the effects of eplerenone.
Pharmacokinetics:
absorption and distribution.
The absolute bioavailability of eplerenone is not known. Maximum plasma concentration is reached approximately 2 h after administration. The maximum plasma concentration (Cmax) and area under the concentration curve (AUC) are proportional to the dose in the range of 10-100 mg and less proportional when used in doses greater than 100 mg. The equilibrium concentration is reached within 2 days. Food intake has no effect on absorption.
Binding of eplerenone to plasma proteins is about 50%, mainly due to binding to alpha 1-acid glycoproteins. The apparent volume of distribution in the equilibrium state is 50 (±7) liters. Eplerenone has no selective binding to erythrocytes.
Metabolism and excretion.
The metabolism of eplerenone is carried out by the CYP3A4 isoenzyme of cytochrome P-450. Active metabolites of eplerenone have not been identified in human plasma.
Less than 5% of the dose of eplerenone is excreted unchanged in the urine and feces. After a single oral dose of the drug labeled with the radioactive isotope, about 32% of the dose was excreted in the feces and about 67% in the urine. The elimination phase elimination half-life of eplerenone is about 3-5 hours. Clearance in blood plasma is about 10 l/h.
Special groups of patients.
Age, sex and race:
The pharmacokinetics of eplerenone in men and women were not significantly different. Equilibrium Cmax (22%) and AUC (45%) were increased in elderly patients compared to younger patients (18-45 years). Equilibrium Cmax and AUC were decreased by 19% and 26%, respectively, in non-Hispanic individuals.
Renal insufficiency:
The pharmacokinetics of eplerenone were studied in patients with renal failure of varying severity and in patients on hemodialysis. Compared with healthy patients, equilibrium AUC and Cmax increased by 38 and 24%, respectively, in patients with severe renal failure, and decreased by 26 and 3%, respectively, in patients on hemodialysis. No correlation was found between eplerenone plasma clearance and creatinine clearance. Eplerenone is not eliminated by hemodialysis.
Help failure:
Eplerenone has not been studied in patients with severe hepatic impairment, so its use is contraindicated in this group of patients.
Heart failure:
Eplerenone clearance in patients with heart failure is similar to that of healthy individuals.
Indications
Indications
Adjunct to standard beta-blocker therapy to reduce the risk of mortality and cardiovascular morbidity in clinically stable patients with left ventricular ejection fraction (LVEF) ≤40%; to reduce the risk of mortality and cardiovascular morbidity in patients with symptomatic heart failure after recent myocardial infarction.
Pharmacological effect
Pharmacological effect
Espiro is a potassium-sparing diuretic.
Pharmaceutical action: Eplerenone has relative selectivity for mineralocorticoid receptors in humans compared to glucocorticoid, progesterone and androgen receptors, blocks their binding to aldosterone, a key hormone of the renin-angiotensin-aldosterone system (RAAS), which is involved in the regulation of blood pressure and the pathogenesis of cardiovascular diseases.
Eplerenone causes a persistent increase in plasma renin and serum aldosterone levels. Subsequently, renin secretion is suppressed by aldosterone via a feedback mechanism. However, an increase in renin activity or circulating aldosterone levels does not affect the effects of eplerenone.
Pharmacokinetics:
Absorption and distribution.
The absolute bioavailability of eplerenone is not known. The maximum concentration in blood plasma is achieved approximately 2 hours after application. The maximum plasma concentration (Cmax) and area under the concentration curve (AUC) are dose proportional in the range of 10-100 mg and less proportional at doses above 100 mg. Equilibrium concentration is achieved within 2 days. Eating does not affect absorption.
The binding of eplerenone to plasma proteins is about 50%, mainly due to binding to alpha 1-acid glycoproteins. The apparent volume of distribution at steady state is 50 (±7) l. Eplerenone does not bind selectively to red blood cells.
Metabolism and excretion.
Eplerenone is metabolized by the cytochrome P-450 isoenzyme CYP3A4. Active metabolites of eplerenone have not been identified in human plasma.
Less than 5% of the eplerenone dose is excreted unchanged in urine and feces. After a single oral dose of a radiolabeled drug, about 32% of the dose was excreted in the feces and about 67% in the urine. The half-life of the elimination phase of eplerenone is approximately 3-5 hours. Plasma clearance is about 10 l/h.
Special groups of patients.
Age, gender and race:
The pharmacokinetics of eplerenone did not differ significantly between men and women. In elderly patients, steady-state values of Cmax (22%) and AUC (45%) were increased compared to young patients (18-45 years). Steady-state Cmax and AUC values in blacks were reduced by 19 and 26%, respectively.
Kidney failure:
The pharmacokinetics of eplerenone were studied in patients with renal failure of varying severity and in patients on hemodialysis. Compared with healthy patients, steady-state AUC and Cmax increased by 38 and 24%, respectively, in patients with severe renal impairment, and decreased by 26 and 3%, respectively, in patients on hemodialysis. No correlation was found between plasma clearance of eplerenone and creatinine clearance. Eplerenone is not removed by hemodialysis.
Liver failure:
Eplerenone has not been studied in patients with severe hepatic impairment and is therefore contraindicated for use in this group of patients.
Heart failure:
The clearance of eplerenone in patients with heart failure is similar to that in healthy individuals.
Active ingredient
Active ingredient
Eplerenone
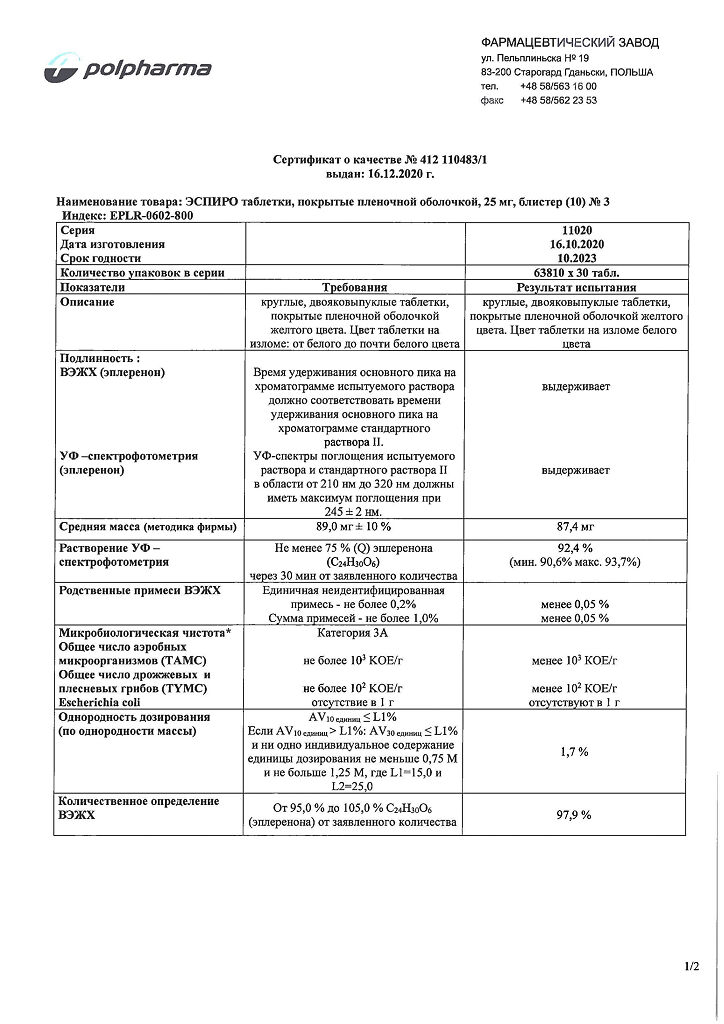
Composition
Composition
Pills
Active ingredient:
eplerenone 25 mg.
Excipients:
lactose monohydrate,
microcrystalline cellulose,
hypromellose,
sodium lauryl sulfate,
croscarmellose sodium,
magnesium stearate,
Shell composition:
Opadry yellow 33G32578, including: hypromellose, titanium dioxide (E171), lactose monohydrate, macrogol, triacetin, iron (III) oxide yellow (E172).
Contraindications
Contraindications
– Hypersensitivity;
– serum potassium level before treatment >5.0 mmol/l;
– moderate to severe renal failure (creatinine clearance < 30 ml/min);– severe liver failure (class C on the Child-Pugh scale);– simultaneous use of potassium-sparing diuretics, potassium supplements or strong CYP3A4 inhibitors (eg, itraconazole, ketoconazole, ritonavir, nelfinavir, clarithromycin, telithromycin and nefazodone);– in combination with angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor antagonists (ARAII);– children under 18 years of age.
Side Effects
Side Effects
Hyperkalemia, dizziness, syncope, hypotension, cough, diarrhea, nausea, constipation, rash, itching, renal dysfunction.
Uncommon (>1/1,000 – pyelonephritis, eosinophilia, hypothyroidism, hyponatremia, dehydration, hypercholesterolemia, hypertriglyceridemia, insomnia, headache, hypoesthesia, myocardial infarction, left ventricular heart failure, atrial fibrillation, tachycardia, thrombosis of the arteries of the lower extremities, orthostatic hypotension, pharyngitis, vomiting, flatulence, increased sweating, back pain, painful cramps of the calf muscles, cholecystitis, gynecomastia, asthenia, malaise, increased levels of urea nitrogen, creatinine, blood glucose, decreased levels of epidermal growth factor receptor.
Frequency not known: angioedema.
Manufacturer
Manufacturer
Polpharma JSC, Poland
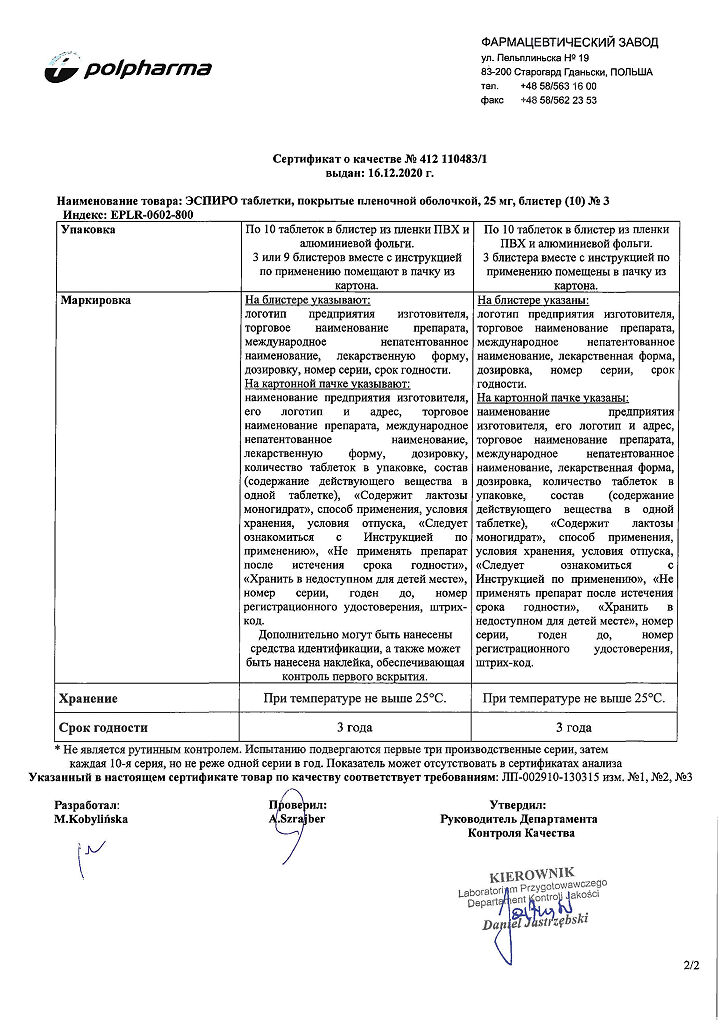
Additional information
| Manufacturer | Polpharma S.A., Poland |
|---|---|
| Medication form | pills |
| Brand | Polpharma S.A. |
Related products
Buy Espiro, 25 mg 30 pcs with delivery to USA, UK, Europe and over 120 other countries.