No products in the cart.
Description
Pharmacotherapeutic group
Allergy medicine – H1-histamine receptor blocker.
ATC code: R06AX27
Pharmacological properties
Pharmacodynamics
Long-acting non-sedative antihistamine drug. It is the primary active metabolite of loratadine. Suppresses histamine release from mast cells. It inhibits the cascade of allergic inflammatory reactions, including release of pro-inflammatory cytokines, including interleukins IL-4, IL-6, IL-8, IL-13, release of adhesion molecules, such as P-selectin. Thus, it prevents and facilitates allergic reactions, has antipruritic and antiexudative properties, decreases capillary permeability, prevents development of tissue edema and smooth muscle spasm.
The drug has no effect on the central nervous system, does not cause somnolence (administration of desloratadine at the recommended dose of 5 mg per day is not associated with an increased incidence of somnolence compared to the placebo group) and does not affect the rate of psychomotor reactions. No prolongation of QT interval on ECG was noted in clinic-pharmacological studies of desloratadine usage in recommended therapeutic dose.
The action of Erius® preparation begins within 30 minutes after oral administration and lasts for 24 hours.
Pharmacokinetics
Desloratadine is well absorbed in gastrointestinal tract. Determined in blood plasma 30 minutes after oral administration. The maximum concentration is reached on average 3 hours after ingestion. It does not penetrate the blood-brain barrier. Binding with plasma proteins is 83-87%. No clinically significant cumulation has been observed in adults and adolescents when administered for 14 days in doses from 5 mg to 20 mg once daily. Simultaneous intake of food or grapefruit juice does not affect the distribution of desloratadine when administered at a dose of 7.5 mg once daily. Desloratadine is not an inhibitor of CYP3A4 and CYP2D6 and is not a substrate or inhibitor of P-glycoprotein. It is intensively metabolized in the liver by hydroxylation to form 3-ON-desloratadine combined with glucuronide. Only small part of oral dose is excreted by kidneys (2 %) and through intestine (7 %) unchanged. The elimination half-life is on average 27 hours.
Indications
Indications
Active ingredient
Active ingredient
Composition
Composition
How to take, the dosage
How to take, the dosage
Interaction
Interaction
Special Instructions
Special Instructions
Synopsis
Synopsis
Contraindications
Contraindications
Side effects
Side effects
Overdose
Overdose
Pregnancy use
Pregnancy use
Similarities
Similarities
Additional information
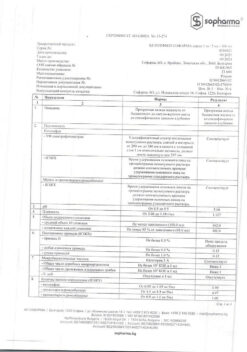
| Shelf life | 2 years. Do not use after the expiration date. |
|---|---|
| Conditions of storage | At a temperature not exceeding 30 ºC. Keep out of reach of children. |
| Manufacturer | Schering-Plough Labo N.V., Belgium |
| Medication form | syrup |
| Brand | Schering-Plough Labo N.V. |
Related products
Buy Erius, syrup 2.5mg/5 ml 60 ml with delivery to USA, UK, Europe and over 120 other countries.