No products in the cart.
Eralfon, 10000 me 1 ml 10 pcs
€1.00
Out of stock
(E-mail when Stock is available)
Description
Eralfon is a treatment for anemia.
Epoetin alpha is a glycoprotein which specifically stimulates erythropoiesis, activates mitosis and maturation of erythrocytes from erythrocyte progenitor cells.
In its composition, biological and immunological properties epoetin alpha is identical to natural human erythropoietin.
The administration of epoetin alfa results in increase of hemoglobin and hematocrit, improvement of blood supply to tissues and heart work.
The most pronounced effect of epoetin alfa is observed in anemia caused by chronic renal failure.
Indications
Indications
– anemia in patients with chronic renal failure, including those on hemodialysis;
– prevention and treatment of anemia in patients with solid tumors, anemia in which was a consequence of anti-tumor therapy;
– prevention and treatment of anemia in patients infected with human immunodeficiency virus (HIV) caused by zidovudine when endogenous erythropoietin levels are less than 500 IU/mL;
– prevention and treatment of anemia in patients with myeloma disease, non-Hodgkin’s lymphoma of low malignancy, chronic lympholeukosis, patients with rheumatoid arthritis;
– treatment and prevention of anemia in premature babies born with low birth weight up to 1.5 kg;
– as part of a preoperative program prior to major surgery in patients with hematocrit levels of 33-39% to facilitate autologous blood collection and reduce the risk associated with the use of allogeneic hemotransfusions if the expected need for transfused blood exceeds the amount that can be obtained by autologous collection without epoetin alfa;
Preceding major surgery with an expected blood loss of 900-1800 mL in adult patients without anemia or with mild to moderate anemia (hemoglobin level 100-130 g/L) to reduce the need for allogeneic hemotransfusions and facilitate recovery of erythropoiesis.
Active ingredient
Active ingredient
Composition
Composition
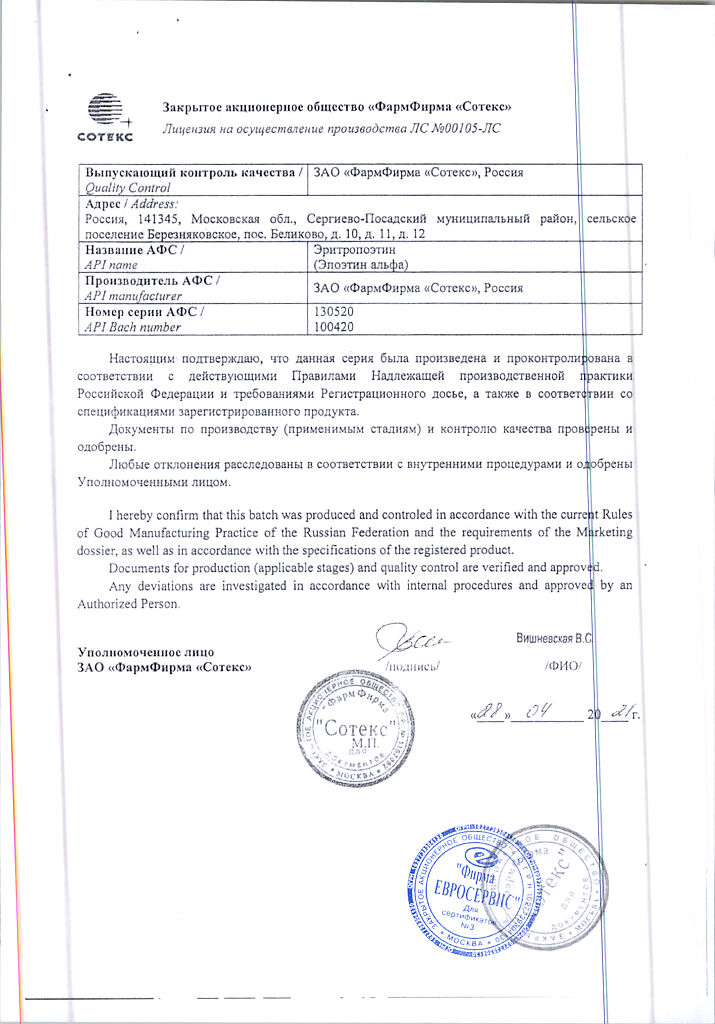
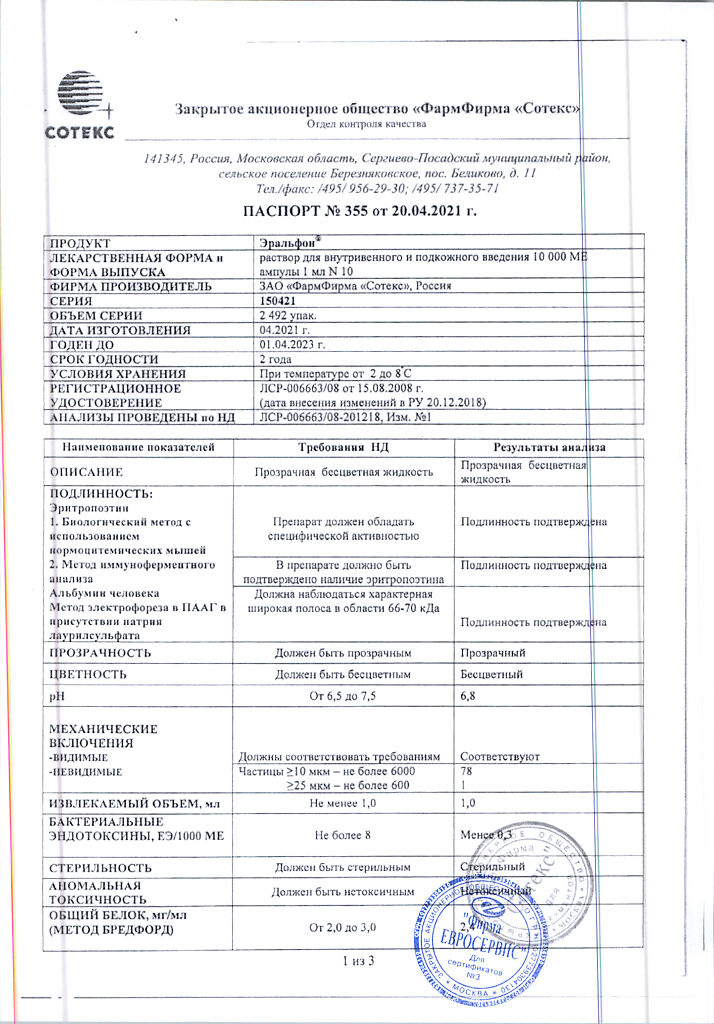
1 ampoule (1 ml) contains as active ingredient Epoetin alfa (REPOETIN-SP – recombinant human erythropoietin) 10000 ME.
Auxiliary substances:
albumin solution,
sodium citrate pentasequihydrate or sodium citrate dihydrate,
How to take, the dosage
How to take, the dosage
The treatment of anemia in patients with chronic renal failure
Adult patients on hemodialysis
Eralfon® is given subcutaneously or intravenously at the end of a dialysis session. When changing the method of administration the drug is administered in the same dose, then the dose is adjusted if necessary (when administering the drug subcutaneously the dose is 20-30% less than when administering it intravenously to achieve the same therapeutic effect). Treatment with the drug includes two stages:
1. The correction phase: When the drug is administered subcutaneously, the initial single dose is 30 IU/kg 3 times per week. When administered intravenously, the initial single dose is 50 IU/kg 3 times weekly. The period of correction lasts till the optimal level of haemoglobin (100-120g/l for adults and 95-110g/l for children) and haematocrit (30-35 %) are reached. These values should be monitored weekly.
The following situations are possible:
1) Hematocrit increases from 0.5% to 1.0% per week. In this case, the dose is not changed until optimal values are reached.
2) Hematocrit increases less than 0.5% per week. In this case it is necessary to increase a single dose 1.5 times.
3) An increase rate of more than 1.0% per week. In this case, a 1.5-fold decrease in the single dose of the drug is necessary.
4) Hematocrit remains low or decreases. The causes of resistance should be analyzed before increasing the dose of the drug.
The efficacy of therapy depends on a properly tailored individual treatment regimen.
The maintenance phase of therapy: in order to maintain hematocrit at 30-35%, the drug dose used in the correction phase should be reduced by 1.5 times. Then the maintenance dose of the drug is chosen individually, taking into account the dynamics of hematocrit and hemoglobin levels.
In children on hemodialysis the initial dose is 50 units/kg 3 times a week. If necessary, the single dose is increased 1 time in 4 weeks by 25 units/kg until optimal hemoglobin concentration is achieved. Maintenance dose in children with body weight less than 10 kg – 75-150 iu/kg (average 100 iu/kg) 3 times a week, 10-30 kg – 60-150 iu/kg (average 75 iu/kg) 3 times a week, over 30 kg – 30-100 iu/kg (average 33 iu/kg) 3 times a week.
In adult pre-dialysis patients, the initial dose is administered subcutaneously or intravenously 3 times 50 IU/kg 3 times per week. If necessary, the single dose is increased once every 4 weeks by 25 IU/kg until optimal hemoglobin concentration is achieved.
The maintenance dose is 17-33 IU/kg 3 times a week.
Prevention and treatment of anemia in patients with solid tumors
Before starting treatment it is recommended to determine endogenous erythropoietin levels. If serum erythropoietin concentration is less than 200 IU/ml, the initial dose of drug in intravenous method of administration is 150 IU/kg 3 times a week. If after 4 weeks of the treatment the haemoglobin level has increased and is more than 10 g/l or the number of reticulocytes has increased by more than 40 000 cells/μl over the basic level, the preparation dose remains the same (150 IU/kg of body weight 3 times a week).
If after 4 weeks of treatment the increase in hemoglobin is less than 10 g/l and the increase in reticulocyte count is less than 40,000 cells/μL compared to baseline, then the dose is increased to 300 IU/kg body weight 3 times per week for the next 4 weeks. If after an additional 4 weeks of 300 IU/kg of therapy the hemoglobin level has increased to at least 10 g/l or the reticulocyte count has increased by more than 40,000 cells/μL, the current dose (300 IU/kg body weight 3 times/week) is maintained. If after 4 weeks of treatment with 300 IU/kg body weight the hemoglobin level increases less than 10 g/l and the reticulocyte count is less than 40,000 cells/μL compared to the initial level, the treatment should be stopped. In case of hemoglobin level increase of more than 20 g/l within a month, the drug dose should be decreased by 25%. If hemoglobin level exceeds 140 g/l, the treatment should be stopped until hemoglobin level drops below 120 g/l and then the drug dose should be continued in 25 % lower than the initial one.
The therapy with the drug should be continued for one month after completion of chemotherapy.
Serum ferritin levels (or serum iron levels) should be determined in all patients before and during treatment with the drug. Additional iron supplementation is prescribed if necessary.
The prevention and treatment of anemia in patients with HIV infection
It is recommended that baseline serum levels of endogenous erythropoietin be determined before starting treatment with Eralfon®. Studies have shown that therapy with erythropoietin levels greater than 500 IU/ml is unlikely to have an effect.
1. correction stage: The drug is given in a dose of 100 IU/kg 3 times weekly subcutaneously or intravenously for 8 weeks. If after 8 weeks of therapy it was not possible to achieve a satisfactory effect (for example, to decrease the necessity of hemotransfusions or increase the hemoglobin level) the dose can be gradually increased (not more often than once in 4 weeks) by 50-100 IU/kg 3 times a week. If it was not possible to reach satisfactory effect from the therapy with Elalfon® in dose of 300 IU/kg 3 times per week, the appearance of response to further therapy in higher doses is unlikely.
2. maintenance therapy phase: after satisfactory effect in anemia correction phase, maintenance dose should provide hematocrit level within 30-35% depending on zidovudine dose changes, presence of concomitant infectious or inflammatory diseases. If hematocrit is greater than 40%, the drug should be discontinued until hematocrit decreases to 36%. During resumption of therapy, the dose of epoetin alfa should be decreased by 25% with subsequent adjustment to maintain the required hematocrit level. Serum ferritin levels (or serum iron levels) should be determined in all patients before and during treatment with the drug. If necessary, additional iron supplementation is prescribed.
Prevention and treatment of anemia in patients with myeloma disease, non-Hodgkin’s lymphoma of low malignancy and with chronic lympholeukosis In these patients the reason of therapy with epoetin alfa is conditioned by inadequate synthesis of endogenous erythropoietin with development of anemia.
Eralfon® is administered subcutaneously at a starting dose of 100 IU/kg three times weekly if the hemoglobin level is below 100 g/l and serum erythropoietin is below 100 IU/ml.
Laboratory control of hemodynamic parameters is performed weekly. If necessary the dose is increased or decreased every 3-4 weeks. If at a weekly dose of 600 IU/kg there is no increase of hemoglobin level further administration of epoetin alfa should be discontinued as ineffective.
Prevention and treatment of anemia in patients with rheumatoid arthritis
In patients with rheumatoid arthritis there is inhibition of endogenous erythropoietin synthesis under the influence of increased concentration of anti-inflammatory cytokines. Treatment of anemia in these patients is carried out by subcutaneous injection of the drug at a dose of 50-75 IU/kg 3 times a week. If hemoglobin level increases less than 10 g/l after 4 weeks of treatment, the drug dose is increased to 150-200 IU/kg 3 times a week. Further increase of the dose seems inexpedient.
The treatment and prevention of anemia in preterm infants who were born with low birth weight
Eralfon® is administered subcutaneously in a dose of 200 IU/kg three times a week from day 6 until the target values of hemoglobin and hematocrit are reached, but not longer than 6 weeks.
Adult patients participating in an autologous blood collection program before surgical interventions
Intravenous administration of the drug is recommended. Epoetin alfa should be administered at the end of the blood collection procedure. All contraindications for autologous blood collection should be considered before prescribing the drug. Before surgical intervention Eralfon® should be administered 2 times a week for 3 weeks. At each visit, a blood sample is taken from the patient (if hematocrit >33% and/or hemoglobin level >110 g/l) and stored for autologous transfusion. The recommended dose of Eralfon® is 600 IU/kg body weight 2 times per week. Serum ferritin level (or serum iron level) should be determined in all patients before and during treatment with the drug. Additional iron supplementation is prescribed if necessary.
If anemia is present, its cause should be determined before starting therapy with epoetin alfa. It is necessary to ensure an adequate intake of iron in the body as soon as possible by prescribing oral iron at a dose of 200 mg/day (based on divalent iron) and to maintain iron intake at this level throughout the course of therapy. Pre- and postoperative patients who do not participate in the autologous blood collection program
The use of subcutaneous administration of the drug at a dose of 600 IU/kg of body weight per week during the 3 weeks preceding surgery (days 21, 14 and 7 before surgery) and on the day of surgery is recommended. If necessary, when it is medically necessary to shorten preoperative period, Eralfon® can be administered daily in a dose of 300 IU/kg of body weight during 10 days before surgery, on the day of surgery and during 4 days after surgery. If preoperative hemoglobin levels reach 150 g/L or higher, epoetin alfa should be discontinued. Before starting therapy with epoetin alfa it is necessary to make sure that patients have no iron deficiency.
All patients should receive adequate amounts of iron (oral 200 mg/day per divalent iron) throughout the course of treatment. If possible, additional oral iron should be provided before therapy with epoetin alfa to ensure adequate iron deposition in the patient.
Interaction
Interaction
Special Instructions
Special Instructions
During treatment, blood pressure should be monitored weekly and general blood counts (including platelets, hematocrit, ferritin) should be performed. In the pre- and postoperative period hemoglobin levels should be monitored more frequently if the baseline was less than 140 g/l. It should be remembered that epoetin alfa in the treatment of anemia does not replace hemotransfusion, but reduces the need for its repeated use.
In patients with a controlled arterial hypertension or history of thrombotic complications it may be necessary to increase the dose of hypotensive drugs and/or anticoagulants respectively. When administered to patients with hepatic impairment, there may be delayed metabolism of epoetin alfa and marked enhancement of erythropoiesis. The safety of using the drug in this category of patients has not been established. Although the drug stimulates erythropoiesis, the possibility of action of epoetin alpha on growth of some types of tumors, including bone marrow, cannot be completely excluded.
We should consider the possibility that preoperative hemoglobin elevation may be a predisposing factor to the development of thrombotic complications. Patients should receive adequate prophylactic antiplatelet therapy prior to elective surgery. In pre- and postoperative period the drug is not recommended to be administered to patients with a baseline hemoglobin level of more than 150 g/l.
In adult patients with chronic renal insufficiency, clinically significant coronary heart disease, or chronic heart failure, hemoglobin levels should not exceed 100-120 g/L.
Before starting treatment, possible causes of inadequate response to the drug (deficiency of iron, folic acid, cyanocobalamin, severe aluminum salts poisoning, concomitant infections, inflammatory processes and injuries, hidden bleeding, hemolysis, bone marrow fibrosis of various etiologies) should be excluded and treatment should be adjusted if necessary.
Before treatment, iron stores in the body should be assessed. In most patients with chronic renal failure, cancer and HIV-infected patients plasma ferritin concentration decreases simultaneously with an increase in hematocrit level. Ferritin concentrations should be determined throughout the course of treatment. If it is less than 100 ng/ml, oral iron replacement therapy at a rate of 200-300 mg/day (100-200 mg/day for children) is recommended. In premature infants, oral iron therapy at a dose of 2 mg/day should be administered as early as possible. Patients who donate autologous blood and are in the pre- or postoperative period should also receive adequate oral iron at a dose of 200 mg/day.
In patients with chronic renal insufficiency, correction of anemia may improve appetite and increase potassium and protein absorption. Periodic correction of dialysis parameters may be necessary to maintain urea, creatinine, and potassium concentrations within normal limits.
In patients with chronic renal insufficiency, serum electrolyte levels should be monitored.
The use of epoetin alfa in predialysis patients has not been reported to accelerate the progression of chronic renal failure. Due to increased hematocrit, an increased dose of heparin is often required during hemodialysis. With inadequate heparinization, occlusion of the dialysis system, vascular access thrombosis is possible, especially in patients with a tendency to hypotension or with complications of arteriovenous fistula (stenosis, aneurysm, etc.). In such patients the prophylaxis of thrombosis is recommended.
When used in women of reproductive age with anemia against a background of chronic renal insufficiency, menstruation may resume. The patient should be warned about possible pregnancy and necessity of using reliable methods of contraception prior to the beginning of therapy. No teratogenic effect was found in experimental studies on rats and rabbits when administered intravenously in doses up to 500 units/kg of body weight per day; a weak statistically insignificant decrease of fertility was noted in higher doses.
At the time of treatment, until the optimal maintenance dose is established, patients with chronic renal insufficiency should be cautious about driving and engaging in other potentially hazardous activities requiring increased concentration and rapid psychomotor reactions (increased risk of increased blood pressure at the beginning of therapy).
Because of the possible more pronounced effect of the drug, its dose should not exceed the dose of recombinant erythropoietin used in the previous course of treatment. For the first two weeks the dose is not changed, the dose/response ratio is evaluated. Thereafter, the dose can be decreased or increased according to the scheme described above.
Contraindications
Contraindications
– hypersensitivity to the drug, or its components;.
– partial red cell aplasia after previous therapy with any erythropoietin;
– uncontrolled arterial hypertension;
– inability to perform adequate anticoagulant therapy;
– in severe occlusive disease of coronary, carotid, cerebral and peripheral arteries and its sequelae, including acute and recently suffered myocardial infarction and acute impairment of cerebral circulation (as part of the preoperative blood collection program before surgical procedures).
With caution:
Malignant neoplasms, epileptic syndrome (including history), thrombocytosis, thrombosis (history), sickle cell anemia, iron-, B12- or folate-deficient states, porphyria, chronic liver failure.
Side effects
Side effects
In the beginning of treatment, flu-like symptoms may be noted: dizziness, somnolence, fever state, headache, myalgia, arthralgia.
Cardiovascular system disorders: dose-dependent increase of blood pressure, worsening of arterial hypertension course (most frequently in patients with chronic renal insufficiency), in some cases – hypertensive crisis, rapid increase of arterial pressure with encephalopathy symptoms (headache, mental confusion) and generalized tonic-clonic convulsions.
Hematopoietic organs: thrombocytosis, in some cases – thrombosis of shunt or arteriovenous fistula (in patients on hemodialysis with . prone to arterial hypotension or with aneurysm, stenosis, etc.), erythrocyte aplasia.
Allergic reactions: skin rash (mild to moderately expressed), eczema, urticaria, pruritus, angioedema.
Local reactions: hyperemia, burning, mild to moderate pain at the injection site (more common with subcutaneous injection).
Laboratory findings: decreased serum ferritin concentration, in uremia – hyperkalemia, hyperphosphatemia.
Others: complications associated with respiratory disorders or decreased blood pressure, immune reactions (induction of antibody formation), exacerbation of porphyria.
Overdose
Overdose
Symptoms: increased side effects.
Treatment: symptomatic. If the hemoglobin level is high – bloodletting.
Pregnancy use
Pregnancy use
Similarities
Similarities
Additional information
| Weight | 0.047 kg |
|---|---|
| Conditions of storage | At a temperature of 2 to 8 ° C. Store out of the reach of children. |
| Manufacturer | PharmFirm Sotex, Russia |
| Medication form | solution |
| Brand | PharmFirm Sotex |
Other forms…
Related products
Buy Eralfon, 10000 me 1 ml 10 pcs with delivery to USA, UK, Europe and over 120 other countries.