No products in the cart.
Equator, tablets 5 mg+20 mg 30 pcs
€26.78 €22.31
Description
Equator has hypotensive, vasodilator and antianginal effects.
Indications
Indications
Essential hypertension (patients who are indicated for combination therapy).
Active ingredient
Active ingredient
Composition
Composition
1 tablet contains
amlodipine 5 mg,
lisinopril 20 mg.
How to take, the dosage
How to take, the dosage
Ingestion, regardless of meals. It is recommended in cases when taking drugs containing separately the active components of Equator in the same doses does not provide proper BP control.
The daily dose for patients not taking antihypertensives is 1 tablet.
2-3 days before therapy, the diuretic should be discontinued if this treatment is given. If diuretic withdrawal is not possible, the initial dose of Equator is 1/2 tablet per day, after which the patient should be monitored by a physician for several hours because of the possible development of symptomatic hypotension.
In cases of heart failure and severe arterial hypertension, the maintenance dose is 1 tablet.
In cases of renal insufficiency, with a creatinine clearance of 30-70 ml/min, 1/2 of the usual starting dose is prescribed because lisinopril is eliminated by the kidneys. Maintenance dose of Equator depends on individual patient’s reaction; treatment requires regular monitoring of renal function, potassium and sodium levels in blood.
Interaction
Interaction
Lisinopril
Potassium-saving diuretics (e.g., spironolactone, amiloride and triamterene), potassium supplements, potassium-containing salt substitutes and other medical drugs that may increase serum potassium levels (e.g., heparin) may lead to hyperkalemia when combined with ACE inhibitors, especially in patients with renal insufficiency and other renal diseases in the history. Serum potassium concentrations should be monitored when prescribing a potassium-affecting drug concomitantly with lisinopril.
Therefore, concomitant administration should be carefully justified and should be performed with extreme caution and regular monitoring of both serum potassium levels and renal function. Potassium-saving diuretics may be taken together with the drug Equator only under medical supervision. If a diuretic is prescribed to a patient receiving Equator, the hypotensive effect is usually enhanced. Therefore, extreme caution should be exercised when taking Equator® in combination with diuretics. Lisinopril mitigates potassium diuretic effect. concomitant use of other antihypertensive drugs may increase the hypotensive effect of the drug Equator.
Concomitant use with nitroglycerin, other nitrates or vasodilators may cause greater BP reduction.
Concomitant use with ACE inhibitors of tricyclic antidepressants/antipsychotics, general anesthetic agents, opioid analgesics: greater BP reduction may be possible.
Ethanol increases the hypotensive effect of the drug. Allopurinol, procainamide, cytostatics or immunosuppressants (systemic GCS) may increase the risk of leukopenia when combined with ACE inhibitors.
Aitacids and colestiramine decrease the bioavailability of ACE inhibitors concomitantly.
Sympathomimetics may decrease the hypotensive effect of ACE inhibitors; the desired effect should be carefully controlled. Concomitant use of ACE inhibitors and hypoglycemic drugs (insulin and hypoglycemic agents for oral administration) may increase the probability of decreased blood glucose concentration and the risk of hypoglycemia. This phenomenon is most often observed during the first week of combined treatment and in patients with renal insufficiency. During long-term use of NSAIDs, including acetylsalicylic acid in high doses, the effectiveness of ACE inhibitors may decrease. Additive effects when taking NSAIDs and ACE inhibitors are manifested by increased serum potassium levels and may lead to worsening of renal function. These effects are usually reversible. Very rarely, acute renal failure may develop, especially in elderly patients and patients in a state of dehydration.
The excretion of lithium may be delayed during concomitant use with ACE inhibitors and therefore serum lithium concentration should be monitored during this period. Co-administration with lithium preparations may increase manifestation of their neurotoxicity (nausea, vomiting, diarrhea, ataxia, tremor, tinnitus).
In concomitant use of ACE inhibitors and gold drugs (sodium aurothiomalate) by IV has described a symptom complex including facial hyperemia, nausea, vomiting and arterial hypotension.
Amlodipine
Studies in elderly patients have shown that diltiazem inhibits the metabolism of amlodipine, probably through inhibition of the CYP3A4 isoenzyme (plasma concentrations are increased by almost 50% and amlodipine effects are increased). It cannot be excluded that stronger inhibitors of CYP3A4 isoenzyme (i.e. ketoconazole, itraconazole, ritonavir) can increase the plasma concentration of amlodipine to a greater extent than diltiazem. Concomitant use should be used with caution.
Concomitant use with inducers of CYP3A4 isoenzyme – with antiepileptic drugs (e.g., carbamazepshum, phenobarbital, phenytoin, fosphenytoin, primidone), rifampicin, herbal medicines containing St. John’s wort may decrease the plasma concentration of amlodipine.
Clinical monitoring with possible adjustment of amlodipine dose during treatment with CYP3A4 isoenzyme inducers and after their withdrawal is indicated. Concomitant use should be used with caution.
. As monotherapy, amlodipine has been well combined with thiazide and “loop” diuretics, general anesthetics, beta-adrenoblockers, ACE inhibitors, long-acting nitrates, sublingual nitroglycerin, Digoxin, warfarin, atorvastatin, sildenafil, antacids (aluminum hydroxide, magnesium hydroxide), simethicone, cimetidine, NSAIDs, antibiotics and oral hypoglycemic drugs.
Amlodipine has no significant effect on the pharmacokinetics of ethanol. Calcium preparations may reduce the effect of slow calcium channel blockers. Amlodipine does not cause significant changes in the pharmacokinetics of cyclosporine. The hypotensive effect of Equator may be reduced when concomitantly administered with estrogens, adrenostimulants. When concomitant use with the drug Equator® procainamide, quinidine and other drugs that prolong the QT interval may contribute to its significant prolongation.
Special Instructions
Special Instructions
Arterial hypotension: marked BP decrease with the development of clinical symptoms may be observed in patients with decreased BOD and/or sodium content due to diuretics, fluid loss or for other reasons, such as profuse sweating, prolonged vomiting and/or diarrhea. In case of arterial hypotension, the patient should be laid down and fluid loss replenished (intravenous infusion of 0.9% sodium chloride solution) if necessary. Preferably, recovery of fluid and/or sodium loss should be performed before starting Equator therapy. BP should be monitored after the initial dose.
Aortic and mitral stenosis: As with all vasodilators, Equator® should be administered with caution in patients with left ventricular outflow tract obstruction and mitral valve stenosis.
Renal dysfunction: in some patients with arterial hypertension without pronounced manifestations of renovascular disease an increase in serum creatinine and urea was observed, in most cases minimal or transient, more pronounced when concomitant use of ACE inhibitors and diuretic. This is most common in patients with a history of renal disease.
The angioedema of the face, extremities, lips, tongue, vocal folds and/or larynx have been reported in patients who have taken an ACE inhibitor, including lisinopril. In these cases, Equator should be stopped immediately and the patient should be closely monitored until the symptoms completely disappear. Swelling of the face, lips and extremities usually goes away on its own, however, antihistamines should be used to reduce the severity of symptoms. Angioedema with laryngeal edema may be fatal. If swelling of the tongue, pharynx or larynx is suspected of causing airway obstruction, emergency measures should be initiated promptly. Appropriate measures include: p/v injection of 0.3-0.5 mg or a slow intravenous injection of 0.1 mg of 0.1% epinephrine (adrenaline) solution, followed by intravenous GCS and antihistamine drugs, and monitoring of vital functions simultaneously.
In patients taking ACE inhibitors, gastrointestinal wall edema was rare. These patients complained of abdominal pain (with or without nausea and vomiting); in some cases, no prior facial edema was observed and C-1 esterase activity was within normal limits. Angioneurotic edema was diagnosed by gastrointestinal computed tomography, or after ultrasound examination, or by surgery; symptoms disappeared after discontinuation of ACE inhibitor. Gastrointestinal wall edema should be included in the differential diagnostic series of abdominal pain in patients taking ACE inhibitors.
Anaphylactic reactions in patients on hemodialysis: cases of anaphylactic shock have been reported in patients undergoing hemodialysis through a polyacrylonitrile membrane (e.g., AN 69) and receiving ACE inhibitors simultaneously, so this combination should be avoided. Patients are advised to use either another type of dialysis membrane or a different class of hypotensive drug.
Anaphylactic reactions in patients during LDL apheresis: Rarely, patients receiving ACE inhibitors during LDL apheresis with dextran sulfate have developed life-threatening anaphylactic reactions. These reactions were prevented by discontinuing ACE inhibitors before each apheresis procedure.
Desensitization with wasp or bee venom: occasionally patients taking ACE inhibitors developed anaphylactic reactions when desensitized with venom of hymenoptera (e.g., wasps or bees). These life-threatening situations can be avoided by timely withdrawal of ACE inhibitors.
Hepatotoxicity: In rare cases, administration of ACE inhibitors has been accompanied by a syndrome that began with cholestatic jaundice or hepatitis and progressed to fulminant liver necrosis and has been fatal in several cases. The mechanism of this syndrome is unclear. Patients receiving Equator® who develop jaundice or have increased hepatic enzyme activity should discontinue Equator with follow-up monitoring.
Hepatic impairment: In patients with hepatic impairment, the T1/2 of amlodipine is prolonged. No dosing recommendations have been developed at this time, so Equator should be prescribed with caution, having previously evaluated the expected benefit and potential risk of treatment.
Hematological toxicity: neutropenia, agranulocytosis, thrombocytopenia and anemia have been reported in rare cases in patients receiving ACE inhibitors. Neutropenia is rare in patients with normal renal function and in the absence of other aggravating factors. Neutropenia and agranulocytosis are reversible and disappear after ACE inhibitor withdrawal. Equator should be used with particular caution in patients with vascular collagenosis, during immunosuppressive therapy, during treatment with allopurinol or procainamide or in combination of these aggravating factors, especially in the presence of previous renal impairment. Some of these patients have developed serious infectious diseases, which in several cases have not been corrected by antibiotic therapy. When prescribing Equator, it is advisable to periodically monitor the white blood cell count in these patients, and to caution them to report the first signs of infectious disease.
Cough: Coughing has often been reported during the use of ACE inhibitors. As a rule, cough is non-productive, persistent and ceased after discontinuation of the drug. When differential diagnosis of cough, cough caused by use of ACE inhibitors should also be considered.
Surgery/general anesthesia: in patients undergoing extensive surgery or during general anesthesia with drugs leading to arterial hypotension, lisinopril may block angiotensin II formation after compensatory renin release. If arterial hypotension develops, probably as a result of the above mechanism, it may be corrected by increasing the RBC.
In elderly patients with impaired renal function, the dose of Equator should be adjusted.
Hyperkalemia: Increased serum potassium levels have been observed in some patients receiving ACE inhibitors. The risk group for development of hyperkalemia includes patients with renal insufficiency, diabetes mellitus, acute heart failure, dehydration, metabolic acidosis or concomitant use of potassium saving diuretics, potassium supplements, potassium containing salt substitutes or any other medical drugs leading to increase in serum potassium level (such as heparin). Serum potassium concentrations should be monitored if concomitant administration with the above drugs is necessary.
Patients of low body weight, short stature, and patients with significant hepatic impairment may require dose reduction.
Equator has no adverse effect on metabolism and plasma lipids and can be used in the treatment of patients with bronchial asthma, diabetes mellitus and gout.
Body weight control and monitoring by a dentist is necessary during treatment (to prevent soreness, bleeding and gum hyperplasia).
Impact on the ability to drive and operate machinery:
Equator may affect the ability to drive vehicles and operate complex machinery. Predominantly at the beginning of treatment transient arterial hypotension and dizziness may occur. Therefore, at the beginning of treatment, patients are advised to avoid driving vehicles, operating machinery and performing other work requiring concentration.
Contraindications
Contraindications
Side effects
Side effects
The adverse reactions that occur are usually mild and transient, and withdrawal of treatment is rarely required.
Side effects caused by the combined drug do not occur more often than in cases of taking each component separately. The most common are: headache (8%), dry cough (5%) and dizziness (3%). Possible: weakness, diarrhea, nausea, vomiting, orthostatic hypotension, skin itching, skin rash, swelling of the ankles of the feet, facial redness, chest pain, arthralgia (1-3%). The frequency of other side effects is less than 1%.
In case of hypersensitivity, angioedema of the face, extremities, lips, tongue, epiglottis and larynx may develop (0.1%). In such cases the treatment should be stopped immediately and the patient should be observed until all symptoms have disappeared.
Laboratory findings: hyperkalemia, increased creatinine, urea nitrogen, liver enzymes activity and blood bilirubin, especially in renal disease, diabetes mellitus and renovascular hypertension.
Hematopoietic organs: leukopenia, neutropenia, agranulocytosis (effect of ACE inhibitor), thrombocytopenia, erythrocytopenia, with long-term treatment a slight decrease of hemoglobin concentration and hematocrit is possible.
Other rare adverse reactions:
Cardiovascular system: arrhythmias, increased palpitations, tachycardia, probably as a result of excessive BP reduction in patients with a high risk of myocardial infarction, cerebrovascular stroke.
Gastrointestinal tract: intestinal dysfunction, dry mouth, abdominal pain, pancreatitis, hepatocellular or cholestatic jaundice, hepatitis, gum hyperplasia, decreased appetite.
Skin disorders: urticaria, increased sweating, itching, alopecia.
Hypersensory system disorders: impaired renal function, frequent urination, oliguria, anuria, acute renal failure, uremia, proteinuria, impotence.
Immune system disorders: syndrome with the appearance of antinuclear antibodies, accelerated sedimentation rate and arthralgia; myalgia; erythema multiforme; fever.
CNS disorders: increased somnolence, muscle fasciculation of extremities and lips, asthenia, mood lability, confusion.
Overdose
Overdose
Symptoms: excessive peripheral vasodilation with a marked decrease in BP, acute vascular failure, impaired water-electrolyte balance, renal failure, hyperventilation, tachycardia, bradycardia, dizziness, anxiety, cough.
The treatment: symptomatic therapy, control of cardiac activity, BP, diuresis and water-electrolyte balance, and its correction, if necessary. If BP is significantly decreased, the patient should be placed in the supine position, lower extremities should be elevated; if there is an unsatisfactory therapeutic response to IV fluid administration, dopamine may be required.
Calcium gluconate may be administered intravenously to stop the effects of amlodipine. If necessary, intravenous administration of angiotensin II. Due to slow absorption of amlodipine in some cases the stomach is washed, activated charcoal is used.
Lisinopril is excreted by hemodialysis; the strong degree of binding to blood proteins makes dialysis of amlodipine ineffective.
Pregnancy use
Pregnancy use
The drug is contraindicated in pregnancy.
If pregnancy is detected, treatment should be discontinued as soon as possible.
Lisinopril administration in the second and third trimesters of pregnancy may cause damage and death of the fetus due to its effect on the kidneys (arterial hypotension, renal failure, hyperkalemia).
Decreased amniotic fluid can lead to skull and facial deformities, impaired limb development, pulmonary hypoplasia and fetal death.
There are no data on such and other exposures earlier in pregnancy.
The drug is contraindicated during lactation due to excretion of amlodipine with breast milk. There are no data indicating penetration of lisinopril into breast milk.
Similarities
Similarities
Additional information
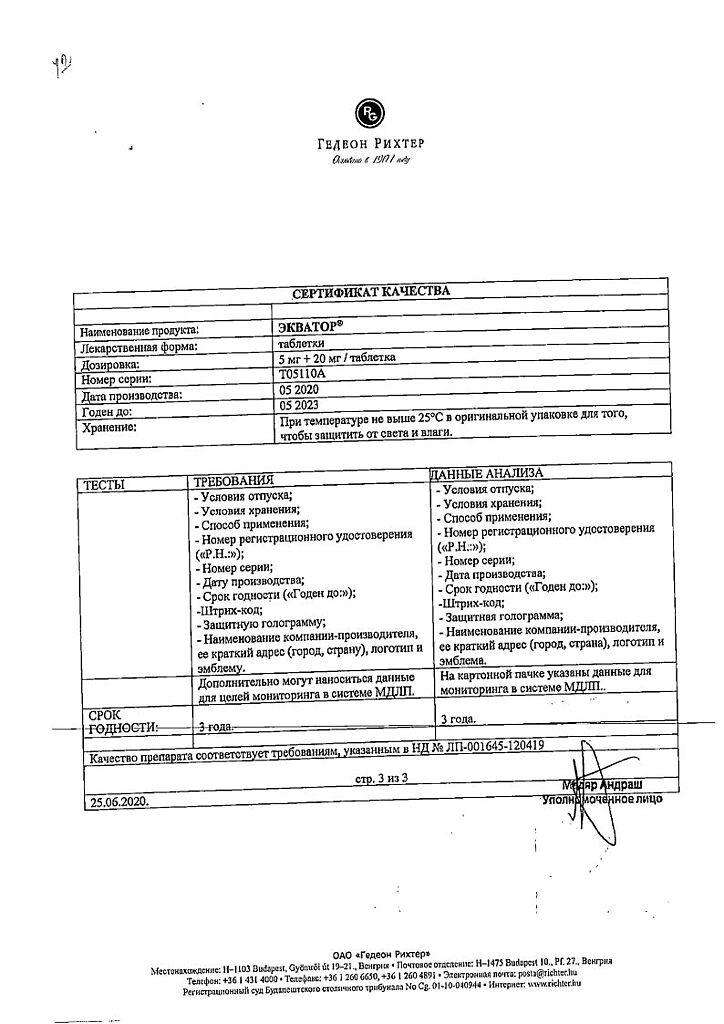
| Shelf life | 2 years |
|---|---|
| Conditions of storage | In a light-protected place, at a temperature not exceeding 30 °C |
| Manufacturer | Gedeon Richter, Hungary |
| Medication form | pills |
| Brand | Gedeon Richter |
Related products
Buy Equator, tablets 5 mg+20 mg 30 pcs with delivery to USA, UK, Europe and over 120 other countries.