No products in the cart.
Enzix Duo, tablets set 2.5mg+10 mg 45 pcs
€13.67 €11.39
Description
ENZIX DUO contains two separate drugs in one package: the angiotensin-converting enzyme (ACE) inhibitor ENALAPRIL and the diuretic INDAPAMID.
Enalapril
Antihypertensive drug, its mechanism of action is related to the reduction of angiotensin I angiotensin II, the reduction of which leads to a direct reduction of aldosterone release.
This decreases total peripheral vascular resistance, systolic and diastolic blood pressure (BP), post- and preload on myocardium.
It dilates arteries more than veins and there is no reflexive increase of heart rate.
Hypotensive effect is more expressed at high level of plasma renin than at normal or reduced level.
Decrease in BP within therapeutic limits does not affect cerebral blood flow, blood flow in the brain vessels is maintained at a sufficient level even at reduced blood pressure.
It increases coronary and renal blood flow. Long-term use reduces left ventricular myocardial hypertrophy and myocyte walls of resistant arteries, prevents the progression of heart failure and slows the development of left ventricular dilatation.
Improves blood supply to ischemic myocardium.
Reduces platelet aggregation. It has some diuretic effect. Enalapril is a “prodrug”: as a result of its hydrolysis enalaprilat is formed which inhibits angiotensin-converting enzyme (ACE).
Time of onset of hypotensive effect when taken orally is 1 hour, it reaches a maximum after 4-6 hours and lasts up to 24 hours.
Indapamide
Hypotensive agent, thiazide-like diuretic of moderate potency and long duration of action, benzamide derivative.
Reduces arterial smooth muscle tone, reduces total peripheral vascular resistance.
It has moderate saluretic and diuretic effects associated with blockade of reabsorption of sodium, chloride, hydrogen and, to a lesser extent, potassium ions in proximal tubules and the cortical segment of the distal tubule of the nephron.
Vasodilator effects and reduction of total peripheral vascular resistance are based on the following mechanisms: reduction of reactivity of the vascular wall to noradrenaline and angiotensin II;
increased synthesis of prostaglandins, having vasodilator activity; inhibition of calcium current in vascular smooth muscle cells. Contributes to the reduction of left ventricular hypertrophy.
In therapeutic doses has no effect on lipid and carbohydrate metabolism (including in patients with concomitant diabetes).
Antihypertensive effect is developed at the end of the first/beginning of the second week if the drug is constantly used and maintained for 24 hours after single use.
Simultaneous use of Enalapril and Indapamide leads to enhancement of antihypertensive effect of Enalapril.
Pharmacokinetics
Enalapril
After oral administration about 60% is absorbed from the gastrointestinal tract. Simultaneous intake of food does not affect the absorption of enalapril. The plasma protein binding of enalaprilat is 50-60%.
Enalapril is rapidly and completely hydrolyzed in the liver to form the active metabolite – enalaprilat, which is a more active ACE inhibitor than enalapril. The bioavailability of the drug is 40%.
Maximum concentration of enalapril in blood plasma is reached after 1-2 hours, enalaprilat – after 3-4 hours.
Enalaprilat easily passes through the histohematic barriers, excluding the blood-brain barrier, a small amount passes through the placenta and into the breast milk.
The elimination half-life of enalaprilat is about 11 hours. Enalapril is mainly excreted through the kidneys 60% (20% as enalapril and 40% as enalaprilate), through the intestine 33% (6% as enalapril and 27% as enalaprilate).
Elimination by hemodialysis (rate 62 ml/min.) and peritoneal dialysis.
Indapamide
After oral administration, it is rapidly and completely absorbed from the gastrointestinal tract; bioavailability is high (93%). Food intake slightly slows down the absorption rate, but does not affect the completeness of absorption.
Maximal concentration in plasma is reached 1-2 hours after oral administration. The equilibrium concentration is reached after 7 days of regular use.
Half-life period is on the average 14-18 hours, the blood plasma protein binding is 79%. It also binds with elastin of vascular smooth muscle.
It has high volume of distribution, passes through histohematic barriers (including placental), penetrates into breast milk.
Metabolized in liver. The kidneys excrete 60 – 80% as metabolites (about 5% is excreted unchanged), through the intestines – 20%.
In patients with renal insufficiency pharmacokinetics is not changed. It does not cumulate.
Indications
Indications
Arterial hypertension.
Pharmacological effect
Pharmacological effect
ENZIX DUO contains two separate medicines in one package: the angiotensin-converting enzyme (ACE) inhibitor ENALAPRIL and the diuretic INDAPAMIDE.
Enalapril
An antihypertensive drug, its mechanism of action is associated with a decrease in the formation of angiotensin II from angiotensin I, a decrease in the content of which leads to a direct decrease in the release of aldosterone.
At the same time, total peripheral vascular resistance, systolic and diastolic blood pressure (BP), post- and preload on the myocardium decrease.
It dilates arteries to a greater extent than veins, while there is no reflex increase in heart rate. Reduces the degradation of bradykinin, increases the synthesis of prostaglandin.
The hypotensive effect is more pronounced with high levels of plasma renin than with normal or reduced levels.
A decrease in blood pressure within therapeutic limits does not affect cerebral circulation; blood flow in the vessels of the brain is maintained at a sufficient level even against the background of reduced blood pressure.
Strengthens coronary and renal blood flow. With long-term use, hypertrophy of the left ventricle of the myocardium and myocytes of the walls of resistant arteries is reduced, prevents the progression of heart failure and slows down the development of left ventricular dilatation.
Improves blood supply to ischemic myocardium.
Reduces platelet aggregation. Has some diuretic effect. Enalapril is a “prodrug”: as a result of its hydrolysis, enalaprilat is formed, which inhibits angiotensin-converting enzyme (ACE).
The onset of the hypotensive effect when taken orally is 1 hour, it reaches a maximum after 4-6 hours and lasts up to 24 hours.
Indapamide
Antihypertensive drug, thiazide-like diuretic with moderate strength and long-lasting action, benzamide derivative.
Reduces the tone of the smooth muscles of the arteries, reduces the total peripheral vascular resistance.
It has moderate saluretic and diuretic effects, which are associated with blockade of the reabsorption of sodium, chlorine, hydrogen, and to a lesser extent potassium ions in the proximal tubules and the cortical segment of the distal tubule of the nephron.
Vasodilating effects and a decrease in total peripheral vascular resistance are based on the following mechanisms: a decrease in the reactivity of the vascular wall to norepinephrine and angiotensin II;
increased synthesis of prostaglandins with vasodilatory activity; inhibition of calcium flow into vascular smooth muscle cells. Helps reduce hypertrophy of the left ventricle of the heart.
In therapeutic doses it does not affect lipid and carbohydrate metabolism (including in patients with concomitant diabetes mellitus).
The antihypertensive effect develops at the end of the first/beginning of the second week with continuous use of the drug and persists for 24 hours with a single dose.
The simultaneous use of Enalapril and Indapamide leads to an increase in the antihypertensive effect of Enalapril.
Pharmacokinetics
Enalapril
After oral administration, about 60% is absorbed from the gastrointestinal tract. Concomitant food intake does not affect the absorption of enalapril. The binding to plasma proteins for enalaprilat is 50-60%.
Enalapril is quickly and completely hydrolyzed in the liver to form an active metabolite, enalaprilat, which is a more active ACE inhibitor than enalapril. Bioavailability of the drug is 40%.
The maximum concentration of enalapril in blood plasma is achieved after 1-2 hours, enalaprilat – after 3-4 hours.
Enalaprilat easily passes through histohematic barriers, excluding the blood-brain barrier, a small amount penetrates the placenta and into breast milk.
The half-life of enalaprilat is about 11 hours. Enalapril is excreted mainly through the kidneys – 60% (20% – in the form of enalapril and 40% – in the form of enalaprilat), through the intestines – 33% (6% – in the form of enalapril and 27% – in the form of enalaprilat).
Removed by hemodialysis (rate 62 ml/min.) and peritoneal dialysis.
Indapamide
After oral administration, it is quickly and completely absorbed from the gastrointestinal tract; bioavailability is high (93%). Eating slightly slows down the rate of absorption, but does not affect the completeness of absorption.
The maximum concentration in blood plasma is achieved 1-2 hours after oral administration. Equilibrium concentration is established after 7 days of regular use.
The half-life averages 14-18 hours, binding to plasma proteins is 79%. It also binds to elastin of smooth muscles of the vascular wall.
It has a high volume of distribution, passes through histohematic barriers (including placental), and penetrates into breast milk.
Metabolized in the liver. 60-80% is excreted by the kidneys in the form of metabolites (about 5% is excreted unchanged) and 20% is excreted through the intestines.
In patients with renal failure, pharmacokinetics do not change. Does not cumulate.
Special instructions
Special instructions
Enalapril
Patients require medical supervision for 2 hours after taking the initial dose of the drug and an additional 1 hour until blood pressure stabilizes.
In patients with a decrease in blood volume (as a result of diuretic therapy, with limited salt intake, hemodialysis, diarrhea, vomiting), when using enalapril (as well as other ACE inhibitors), even at the initial dose, the risk of a sudden and pronounced decrease in blood pressure increases.
Transient arterial hypertension is not a contraindication for continuing treatment with the drug after stabilization of blood pressure. In case of a repeated pronounced decrease in blood pressure, the dose should be reduced or the drug discontinued.
The use of high-strength dialysis membranes increases the risk of developing an anaphylactic reaction. On days free from dialysis, the dosage regimen should be adjusted depending on the blood pressure level.
The condition of patients with severe heart failure, coronary artery disease and cerebrovascular diseases should be carefully monitored.
In such patients, a sharp decrease in blood pressure can lead to myocardial infarction, stroke, or impaired renal function.
Sudden withdrawal of the drug does not lead to a sharp increase in blood pressure.
Before studying the function of the parathyroid glands, enalapril should be discontinued.
If side effects or angioedema develop, the drug should be discontinued and appropriate treatment prescribed.
Before surgery (including dentistry), the patient should warn the surgeon/anesthesiologist about the use of ACE inhibitors.
Before and during treatment with ACE inhibitors, periodic monitoring of blood pressure, blood parameters (hemoglobin, potassium, creatinine, urea, liver transaminase activity), and protein in the urine is necessary.
Indapamide
When prescribing indapamide to patients taking cardiac glycosides, laxatives, against the background of hyperaldosteronism, as well as elderly patients, regular monitoring of potassium and creatinine levels is indicated.
While taking indapamide, the concentration of potassium, sodium, magnesium in the blood plasma, pH, concentration of glucose, uric acid and residual nitrogen should be systematically monitored.
The most careful monitoring is indicated for liver cirrhosis (especially with edema or ascites – the risk of developing metabolic alkalosis, which increases the manifestations of hepatic encephalopathy), coronary artery disease, heart failure, as well as in elderly patients.
The high-risk group also includes patients with an increased QT interval on the ECG (congenital or developed against the background of any pathological process).
The first determination of potassium concentration in the blood should be carried out during the first week of treatment.
Hypercalcemia while taking indapamide may be a consequence of previously undiagnosed hyperparathyroidism.
In patients with diabetes mellitus, it is extremely important to control blood glucose levels, especially in the presence of hypokalemia.
Significant dehydration can lead to the development of acute renal failure (decreased glomerular filtration rate). Patients need to compensate for water loss and carefully monitor renal function at the beginning of treatment.
Indapamide may give a positive result during a doping test.
In patients with arterial hypertension and hyponatremia (due to taking diuretics), it is necessary to stop taking diuretics 3 days before starting ACE inhibitors (if necessary, diuretic use can be resumed a little later), or in such cases, initial low doses of ACE inhibitors are prescribed.
When prescribing indapamide, it should be taken into account that sulfonamide derivatives can aggravate the course of SLE.
Use in pediatrics
The effectiveness and safety of enalapril and indapamide in children and adolescents under 18 years of age have not been established.
Impact on the ability to drive vehicles and operate machinery
At the beginning of treatment, until the completion of the dose selection period, the patient should refrain from driving vehicles and engaging in potentially hazardous activities that require increased
concentration of attention and speed of psychomotor reactions, because Dizziness is possible, especially after taking the initial dose of the drug.
Active ingredient
Active ingredient
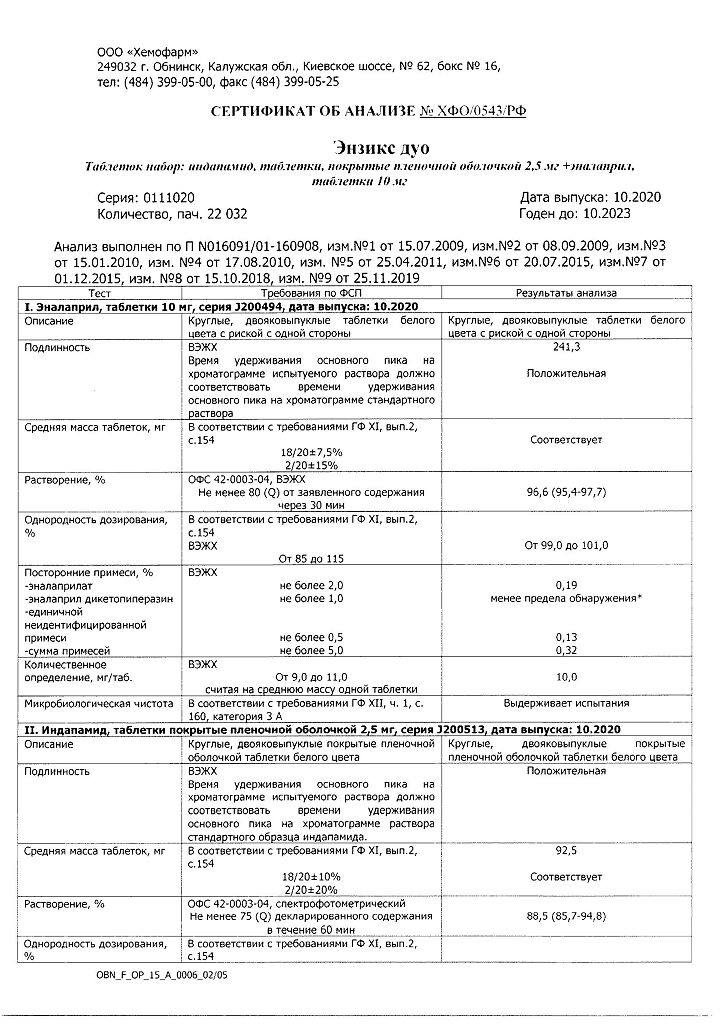
Indapamide, Enalapril
Composition
Composition
1 tablet of enalapril contains:
Active ingredient:
Enalapril maleate – 10,000 mg;
Excipients:
Lactose monohydrate – 125,000 mg,
Magnesium carbonate – 84,600 mg,
Gelatin – 9,200 mg,
Crospovidone – 9,200 mg,
Magnesium stearate – 2,000 mg.
1 tablet of indapamide contains:
Active ingredient:
Indapamide 2.5000 mg;
Excipients:
Lactose monohydrate – 76.9600 mg,
Povidone-K30 – 2.8200 mg,
Crospovidone – 0.8800 mg,
Magnesium stearate – 0.8800 mg,
Sodium lauryl sulfate – 0.4400 mg,
Talc – 3.5200 mg;
Shell composition:
Hypromellose – 1.7222 mg,
Macrogol 6000 – 0.3445 mg,
Talc – 1.9030 mg,
Titanium dioxide E 171 – 0.4303 mg.
Pregnancy
Pregnancy
The drug is contraindicated for use during pregnancy and lactation. If it is necessary to use the drug Enzix during lactation, breastfeeding should be discontinued.
For newborns and infants who have been exposed in utero to ACE inhibitors, it is recommended to conduct careful monitoring for timely detection of a pronounced decrease in blood pressure, oliguria,
hyperkalemia and neurological disorders, the development of which is possible due to a decrease in renal and cerebral blood flow with a decrease in blood pressure caused by ACE inhibitors.
In oliguria, it is necessary to maintain blood pressure and renal perfusion by administering appropriate fluids and vasoconstrictors.
Contraindications
Contraindications
Enzix Duo contains lactose monohydrate. The drug should not be used in patients with lactose intolerance, lactase deficiency, or glucose-galactose malabsorption.
Enalapril
Hypersensitivity to enalapril and other ACE inhibitors, a history of angioedema associated with treatment with ACE inhibitors, hereditary or idiopathic angioedema,
simultaneous use with aliskiren and/or aliskiren-containing drugs in patients with diabetes mellitus and/or moderate or severe renal impairment (glomerular filtration rate (GFR)
less than 60 ml/min/1.73 m2), pregnancy, breastfeeding, age under 18 years (efficacy and safety have not been established).
Use with caution in primary hyperaldosteronism, bilateral renal artery stenosis, conditions accompanied by a decrease in circulating blood volume (BCV) (including vomiting, diarrhea),
stenosis of the artery of a single kidney, hyperkalemia, condition after kidney transplantation; aortic stenosis, mitral stenosis (with impaired hemodynamic parameters), hypertrophic obstructive cardiomyopathy,
renovascular hypertension, systemic connective tissue diseases (systemic lupus erythematosus, scleroderma, etc.), coronary heart disease, cerebrovascular diseases, diabetes mellitus,
renal failure (proteinuria more than 1 g/day), liver failure, a history of allergy or angioedema in patients on a diet with
restriction of table salt or those on hemodialysis; when taken simultaneously with immunosuppressants, saluretics, potassium-sparing diuretics, potassium preparations, potassium-containing
salt substitutes and lithium preparations, during the procedure of low-density lipoprotein apheresis (LDL apheresis) using dextran sulfate, during desensitization with poison
Hymenoptera insects; in patients of the Negroid race; in patients after major surgery or during general anesthesia; in patients undergoing dialysis using
high-flow membranes (such as AN 69®); in elderly patients (over 65 years old).
Indapamide
Hypersensitivity to the drug, other sulfonamide derivatives or other components of the drug, anuria, refractory hypokalemia, severe liver failure (including encephalopathy)
and/or severe renal failure (creatinine clearance less than 30 ml/min), pregnancy, breastfeeding, age under 18 years (efficacy and safety have not been established).
Prescribed with caution for diabetes mellitus in the stage of decompensation, hyperuricemia (especially accompanied by gout and urate nephrolithiasis), impaired liver and kidney function, water-electrolyte balance disorders, hyperparathyroidism;
in weakened patients or in patients receiving concomitant therapy with other antiarrhythmic drugs, while taking drugs that prolong the QT interval.
Side Effects
Side Effects
Enalapril
From the central nervous system and peripheral nervous system: headache, dizziness, weakness, insomnia, anxiety, confusion, fatigue, drowsiness (2-3%); in some cases, when used in high doses – nervousness, depression, paresthesia.
From the respiratory system: nonproductive dry cough, interstitial pneumonitis, bronchospasm/bronchial asthma, shortness of breath, rhinorrhea, pharyngitis.
From the senses: disorders of the vestibular apparatus, hearing and vision impairment, tinnitus.
From the digestive system: dry mouth, anorexia, dyspeptic symptoms (nausea, diarrhea or constipation, vomiting, abdominal pain), intestinal obstruction, pancreatitis, impaired liver function and
biliary excretion, hepatitis (hepatocellular or cholestatic), jaundice, increased activity of liver transaminases, hyperbilirubinemia.
From the cardiovascular system: excessive decrease in blood pressure, orthostatic collapse; rarely – chest pain, angina pectoris, myocardial infarction (usually associated with a pronounced decrease in blood pressure),
arrhythmias (atrial brady- or tachycardia, atrial fibrillation), palpitations, thromboembolism of the branches of the pulmonary artery, pain in the heart, fainting.
Metabolic: hyperkalemia, hyponatremia, hypoglycemia (in patients with diabetes).
From the hematopoietic system: rarely – decreased hematocrit and hemoglobin concentration, thrombocytopenia, neutropenia, agranulocytosis (in patients with autoimmune diseases), eosinophilia, increased ESR.
From the urinary system: impaired renal function, proteinuria, hypercreatininemia, increased urea content.
From the reproductive system: decreased libido, hot flashes, decreased potency.
Dermatological reactions: alopecia, photosensitivity.
Allergic reactions: skin rash, angioedema of the face, limbs, lips, tongue, glottis and/or larynx, dysphonia, erythema multiforme, exfoliative dermatitis, Stevens-Johnson syndrome,
toxic epidermal necrolysis, pemphigus, itching, urticaria, serositis, vasculitis, myositis, arthralgia, arthritis, stomatitis, glossitis; very rarely – intestinal angioedema.
Indapamide
From the central nervous system and peripheral nervous system: asthenia, nervousness, headache, dizziness, drowsiness, vertigo, insomnia, depression, paresthesia; rarely – increased fatigue, general weakness, malaise, muscle spasms, tension, irritability, anxiety.
From the digestive system: possible nausea, anorexia, dry mouth, gastralgia, vomiting, diarrhea or constipation, discomfort in the abdomen, pancreatitis.
From the senses: conjunctivitis, visual impairment.
From the respiratory system: cough, pharyngitis, sinusitis, rhinorrhea; rarely – rhinitis.
From the cardiovascular system: orthostatic hypotension, ECG changes characteristic of hypokalemia, arrhythmias, palpitations.
From the urinary system: increased frequency of infections, nocturia, polyuria, increased urea nitrogen in the blood plasma, hypercreatininemia.
Metabolism: hypokalemia, hyponatremia, hypochloremic alkalosis, hypercalcemia, glycosuria, sweating, weight loss.
From the reproductive system: decreased potency, decreased libido.
Allergic reactions: skin rash, urticaria, itching, hemorrhagic vasculitis.
Other: flu-like syndrome, chest pain, back pain, infections, exacerbation of SLE.
Interaction
Interaction
Enalapril
The simultaneous use of enalapril and indapamide leads to an increase in the antihypertensive effect of enalapril.
With simultaneous use of enalapril with NSAIDs, including selective COX-2 inhibitors, analgesics and antipyretics, the hypotensive effect of enalapril may be reduced.
In some cases, in patients with impaired renal function receiving NSAIDs, including selective COX-2 inhibitors, the use of ACE inhibitors may lead to a further deterioration of renal function. These changes are reversible.
The antihypertensive effect of enalapril is enhanced by diuretics, beta-blockers, methyldopa, nitrates, slow calcium channel blockers of the dihydropyridine series, hydralazine, prazosin.
The use of enalapril in combination with potassium-sparing diuretics (spironolactone, triamterene, amiloride), as well as with potassium-containing drugs, increases the risk of hyperkalemia.
Enalapril weakens the effect of drugs containing theophylline.
Immunosuppressants, allopurinol, and cytostatics increase the hematotoxicity of enalapril. Drugs that cause bone marrow suppression increase the risk of developing neutropenia and/or agranulocytosis.
Enalapril helps slow down the excretion of lithium (with simultaneous use of enalapril with lithium salts, monitoring the concentration of lithium in the blood plasma is indicated).
The combined use of ACE inhibitors and hypoglycemic agents (insulin, oral hypoglycemic drugs) may enhance the hypoglycemic effect of the latter with the risk of developing hypoglycemia.
This is most common during the first 3 weeks of co-use and in patients with renal impairment.
In patients with diabetes mellitus receiving oral hypoglycemic agents and insulin, monitoring of blood glucose levels is necessary, especially during the first month of co-administration with ACE inhibitors.
A complex of symptoms, including facial flushing, nausea, vomiting and hypotension, has been described in rare cases with the combined use of parenteral gold preparations (sodium aurothiomalate) and ACE inhibitors (enalapril).
Ethanol enhances the hypotensive effect of enalapril.
Indapamide
With simultaneous use of indapamide with saluretics, cardiac glycosides, gluco- and mineralocorticoids, tetracosactide, amphotericin B (intravenous), laxatives, the risk of developing hypokalemia increases.
When taking indapamide simultaneously with cardiac glycosides, the likelihood of developing glycoside intoxication increases; with calcium supplements – hypercalcemia; with metformin – possible worsening of lactic acidosis.
Indapamide helps to slow down the excretion of lithium and thereby increase its concentration in the blood plasma.
Astemizole, erythromycin (intravenously), pentamidine, sultopride, terfenadine, vincamine, antiarrhythmic drugs of class I A (quinidine, disopyramide) and class III (amiodarone, bretylium, sotalol) when taken against the background of idapamide can lead to the development of pirouette-type arrhythmia.
NSAIDs, corticosteroids, tetracosactide, sympathomimetics reduce the hypotensive effect of indapamide; baclofen – enhances.
The combination of indapamide with potassium-sparing diuretics may be effective in some categories of patients, however, the possibility of developing hypo- or hyperkalemia is not completely excluded, especially in patients with diabetes mellitus and renal failure.
ACE inhibitors, when used simultaneously with indapamide, increase the risk of developing arterial hypotension and/or acute renal failure (especially with existing renal artery stenosis).
Indapamide increases the risk of developing renal dysfunction when used simultaneously with iodine-containing contrast agents in high doses (dehydration).
Before using iodinated contrast media in patients taking indapamide, it is necessary to restore fluid loss.
Tricyclic antidepressants and antipsychotic drugs enhance the hypotensive effect of indapamide and increase the risk of developing orthostatic hypotension.
When indapamide is used concomitantly with cyclosporine, the risk of developing hypercreatininemia increases.
Indapamide reduces the effect of indirect anticoagulants (coumarin or indanedione derivatives) due to an increase in the concentration of coagulation factors as a result of a decrease in blood volume and an increase in their production by the liver (dose adjustment may be required).
Indapamide enhances the effect of non-depolarizing muscle relaxants.
Overdose
Overdose
Enalapril
Symptoms:
a pronounced decrease in blood pressure up to the development of collapse, myocardial infarction, acute cerebrovascular accident or thromboembolic complications, convulsions, stupor.
Treatment:
the patient is transferred to a horizontal position with a low headboard. In mild cases, gastric lavage and ingestion of a saline laxative are indicated.
In more severe cases, measures are taken to stabilize blood pressure: intravenous administration of saline, plasma expanders, angiotensin II, and possible hemodialysis.
Indapamide
Symptoms: nausea, vomiting, weakness, gastrointestinal dysfunction, water and electrolyte disturbances; in some cases – excessive decrease in blood pressure, dizziness, drowsiness, confusion, respiratory depression. Patients with liver cirrhosis may develop hepatic coma.
Treatment: gastric lavage and/or administration of activated carbon, correction of water and electrolyte balance, symptomatic therapy. There is no specific antidote.
Storage conditions
Storage conditions
In a dry place, at a temperature of 15–25 °C
Shelf life
Shelf life
3 years
Manufacturer
Manufacturer
Hemofarm LLC, Russia
Additional information
| Shelf life | 3 years |
|---|---|
| Conditions of storage | In a dry place, at 15-25 °C |
| Manufacturer | Chemopharm LLC, Russia |
| Medication form | tablet set |
| Brand | Chemopharm LLC |
Related products
Buy Enzix Duo, tablets set 2.5mg+10 mg 45 pcs with delivery to USA, UK, Europe and over 120 other countries.