No products in the cart.
Enterol, capsules 250 mg blister 30 pcs
€23.40 €19.50
Description
Pharmacotherapeutic group: Antidiarrheal.
ATX code: A07FA02
Pharmacological properties:
Pharmacodynamics
Saccharomyces boulardii. CNCM I-745 is a live non-pathogenic yeast (deposited by the National Collection of Cultures and Microorganisms (CNCM), International Depository, Institut Pasteur, Paris).
The drug Saccharomyces boulardii is a probiotic and acts in the gastrointestinal tract as an anti-diarrheal microorganism.
The pharmacodynamics of Saccharomyces boulardii have been studied in various models in in
– has an antimicrobial effect due to its antagonistic effect against pathogenic and opportunistic microorganisms: Clostridium difficile, Candida albicans, Candida krusei, Candida pseudotropicalis, Klebsiella pneumoniae, Pseudomonas aeruginosa, Salmonella typhimurium, Yersinia enterocolitica, Escherichia coli, Shigella dysenteriae, Staphylococcus aureusand others, as well as Entamoeba histolytica and Lambliae;
–has antitoxic effects against bacterial cyto- and enterotoxins;
– increases intestinal enzymatic function;
A component of the cell wall of Saccharomyces boulardii mannitol is a substrate for pathogenic strains of Escherichia coli and Salmonella typhimuriumwhich causes their adhesion (attachment) to the surface of Saccharomyces boulardii and subsequent elimination from the body;
has a natural resistance to antibiotics.
Pharmacokinetics
Saccharomyces boulardii is not eubiotic, is not part of the microflora of the healthy human body. Saccharomyces boulardii is not absorbed, passes through the digestive tract unchanged, without colonization. The concentration of Saccharomyces boulardii in the intestine is maintained at a constant level during the period of administration of the drug. Saccharomyces boulardii is completely eliminated from the body within 2-5 days after discontinuation of the drug.
Indications
Indications
Treatment and prevention of diarrhea of any etiology in adults and children over 1 year of age, including:
– intestinal dysbacteriosis (dysbiosis);
– acute infectious, viral or bacterial diarrhea;
– diarrhea caused by taking antibiotics (antibiotic-associated diarrhea);
– irritable bowel syndrome, enterocolitis;
– traveler’s diarrhea;
– diarrhea caused by Clostridium difficile, in combination with vancomycin or metronidazole therapy;
– adverse reactions from the gastrointestinal tract during Helicobacter pylori eradication therapy.
Pharmacological effect
Pharmacological effect
Pharmacotherapeutic group: Antidiarrheal agent.
ATX code: A07FA02
Pharmacological properties:
Pharmacodynamics
Saccharomyces boulardii CNCM I-745 is a live, non-pathogenic yeast (deposited by the National Collection of Cultures and Microorganisms (CNCM), International Depository, Institut Pasteur, Paris).
The drug Saccharomyces boulardii is a probiotic and acts in the gastrointestinal tract as an antidiarrheal microorganism.
The pharmacodynamics of Saccharomyces boulardii was studied in various models during in vitro and in vivo studies, as well as during preclinical and clinical studies, which showed that the drug:
– has an antimicrobial effect due to an antagonistic effect against pathogenic and opportunistic microorganisms: Clostridium difficile, Candida albicans, Candida krusei, Candida pseudotropicalis, Klebsiella pneumoniae, Pseudomonas aeruginosa, Salmonella typhimurium, Yersinia enterocolitica, Escherichia coli, Shigella dysenteriae, Staphylococcus aureus and others, as well as Entamoeba histolytica and Lambliae;
– has an antitoxin effect against bacterial cyto- and enterotoxins;
– increases the enzymatic function of the intestines;
– a component of the cell wall of Saccharomyces boulardii, mannitol is a substrate for pathogenic strains of Escherichia coli and Salmonella typhimurium, which determines their adhesion (attachment) to the surface of Saccharomyces boulardii and subsequent removal from the body;
– has natural resistance to antibiotics.
Pharmacokinetics
Saccharomyces boulardii is not a eubiotic and is not part of the microflora of a healthy human body. Saccharomyces boulardii is not absorbed and passes through the digestive tract unchanged, without colonization. The concentration of Saccharomyces boulardii in the intestine is maintained at a constant level during the period of use of the drug. Saccharomyces boulardii is completely eliminated from the body within 2-5 days after stopping the drug.
Special instructions
Special instructions
Diarrhea may be a symptom of a more serious underlying condition.
If diarrhea persists after two days of taking the drug, as well as if the temperature rises, blood or mucus is detected in the stool, treatment should be reconsidered and the possibility of oral or parenteral rehydration should be considered. For children under 2 years of age, consultation with the attending physician is recommended.
After diarrhea stops, treatment with the drug can be continued for several days.
The use of the drug does not replace rehydration if necessary. Rehydration doses and route of administration should be selected depending on the severity of diarrhea and the age and condition of the patient.
In children aged 1 to 2 years: Consultation with a physician may be required due to the possibility of an underlying condition. Rehydration may be the mainstay of treatment for acute diarrhea in children and should be systematically reviewed.
In children aged 2 to 6 years: Rehydration may be the mainstay of treatment for acute diarrhea in children and should be systematically reviewed. Oral rehydration is given to prevent or treat dehydration. It is recommended to use ready-made preparations provided for this purpose.
In cases of severe or prolonged diarrhea, severe vomiting, or refusal of oral rehydration, parenteral rehydration should be considered.
In adults and children over 6 years of age: If diarrhea persists after two days of taking the drug, treatment should be reconsidered and oral or parenteral rehydration should be considered.
Due to the yeast nature of the drug Enterol®, very rarely cases of fungemia have been reported (Saccharomyces cultures have been isolated from the blood), mainly in patients who have had a central venous catheter installed, in patients who are in a state of extreme severity or have immunodeficiency conditions, which is often accompanied by fever (increased body temperature). In most cases, the outcome was satisfactory after discontinuation of treatment, administration of antifungal therapy and removal of the catheter, if necessary. However, in some patients whose condition was severe, the outcome was unfavorable.
Particular care must be taken when working with the drug in the presence of patients with installed central and peripheral venous catheters, even if they are not receiving treatment with Enterol®, measures must be taken to eliminate the risk of contamination of the drug through contact with contaminated hands or the spread of microorganisms by airborne droplets.
It is recommended that patients prepare for taking the drug and open Enterol® capsules wearing medical gloves; in case of contact with the contents of the capsules, the gloves must be disposed of immediately after use, hands must be thoroughly washed.
Impact on the ability to drive vehicles and machinery:
The use of the drug Enterol® does not affect the ability to drive vehicles or engage in other potentially hazardous activities that require increased concentration and speed of psychomotor reactions.
Active ingredient
Active ingredient
Saccharomyces boulardii
Composition
Composition
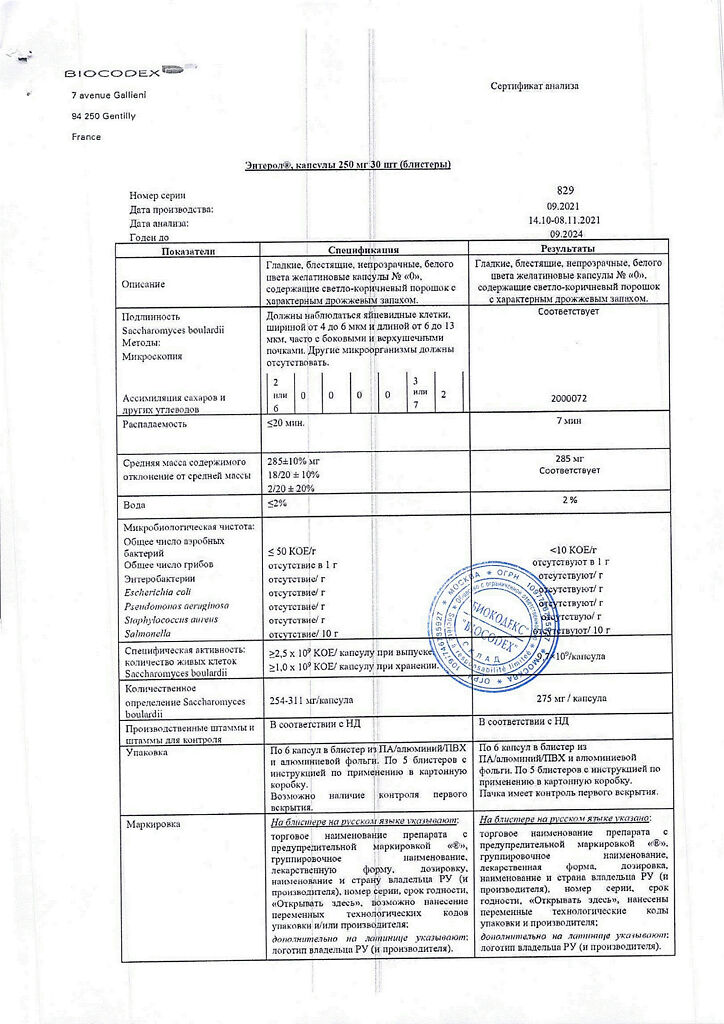
Active ingredient: Saccharomyces boulardii CNCM I-745 lyophilisate 250 mg.
Excipients: lactose monohydrate, magnesium stearate.
Capsule: titanium dioxide, gelatin.
Pregnancy
Pregnancy
There are no or limited data on the use of Saccharomyces boulardii in pregnant women and during breastfeeding.
Saccharomyces boulardii is not absorbed from the gastrointestinal tract.
As a precautionary measure, it is preferable to assess the ratio of the expected benefit for the mother to the potential risk of use for the fetus and child before using the drug during pregnancy and breastfeeding.
You should consult your doctor.
Contraindications
Contraindications
Hypersensitivity to one of the components of the drug, allergy to yeast, primarily to Saccharomyces boulardii.
Presence of a central venous catheter, patients in serious condition or with severe immunocompromise due to the risk of fungemia.
Lactose intolerance, lactase deficiency, congenital galactosemia,
syndrome
glucose-galactose malabsorption.
Children under 1 year of age.
Side Effects
Side Effects
Skin and subcutaneous tissue disorders
Very rare: allergic reactions – itching, papular rash (urticaria), skin rash, local or widespread throughout the body (local or generalized exanthema), swelling of the face (angioedema).
Immune system disorders
Very rare: anaphylactic reactions or anaphylactic shock.
Gastrointestinal disorders
Rarely: flatulence;
Frequency unknown: constipation.
Infectious and parasitic diseases
Very rare: fungemia (in patients who have a central venous catheter installed, as well as in hospitalized patients, people with immunodeficiency conditions (see section “Special Instructions”)).
Interaction
Interaction
Enterol is not taken together with antifungal drugs.
Compatible with antibiotics.
Overdose
Overdose
An overdose of the drug is not possible, due to its pharmacokinetic properties.
Storage conditions
Storage conditions
At a temperature of 15-25ºС, out of the reach of children.
Shelf life
Shelf life
3 years. Do not use after the date indicated on the package.
Manufacturer
Manufacturer
Biocode, France
Additional information
| Shelf life | 3 years. Do not use after the date on the package. |
|---|---|
| Conditions of storage | At 15-25ºC, out of the reach of children. |
| Manufacturer | Biocodex, France |
| Medication form | capsules |
| Brand | Biocodex |
Other forms…
Related products
Buy Enterol, capsules 250 mg blister 30 pcs with delivery to USA, UK, Europe and over 120 other countries.