No products in the cart.
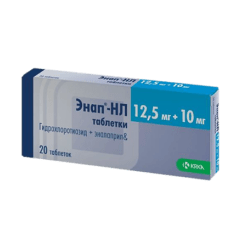
Enap-NL 20, tablets 12.5mg+20 mg 20 pcs
€11.55 €10.11
Description
A combination drug, the action of which is due to the properties of its constituent components.
Enalapril is an ACE inhibitor and is a prodrug: its hydrolysis produces enalaprilate, which inhibits ACE.
Hydrochlorothiazide is a thiazide diuretic. It acts at the level of the distal renal tubules, increasing the excretion of sodium and chlorine ions.
In the beginning of treatment with hydrochlorothiazide, the volume of fluid in the vessels, decreases as a result of increased sodium and fluid excretion, which leads to a decrease in BP and a decrease in cardiac output.
As a consequence of hyponatremia and decreased body fluid, the renin-angiotensin-aldosterone system is activated. Reactive increase of angiotensin II concentration partially limits BP reduction. If therapy is continued, the hypotensive effect of hydrochlorothiazide is based on the reduction of total peripheral vascular resistance .
The activation of renin-angiotensin-aldosterone system results in metabolic effects on blood electrolyte balance, uric acid, glucose and lipids that partially neutralize the effectiveness of antihypertensive treatment.
Indications
Indications
Arterial hypertension.
chronic heart failure.
Pharmacological effect
Pharmacological effect
A combined drug, the effect of which is determined by the properties of the components included in its composition.
Enalapril is an ACE inhibitor and is a prodrug: as a result of its hydrolysis, enalaprilat is formed, which inhibits ACE.
Hydrochlorothiazide is a thiazide diuretic. Acts at the level of the distal renal tubules, increasing the excretion of sodium and chloride ions.
At the beginning of treatment with hydrochlorothiazide, the volume of fluid in the vessels decreases as a result of increased excretion of sodium and fluid, which leads to a decrease in blood pressure and a decrease in cardiac output.
Due to hyponatremia and decreased fluid in the body, the renin-angiotensin-aldosterone system is activated. A reactive increase in angiotensin II concentration partially limits the decrease in blood pressure. With continued therapy, the hypotensive effect of hydrochlorothiazide is based on a decrease in total peripheral vascular resistance.
Activation of the renin-angiotensin-aldosterone system results in metabolic effects on blood electrolyte balance, uric acid, glucose and lipids, which partially counteract the effectiveness of antihypertensive treatment.
Special instructions
Special instructions
Impact on the ability to drive vehicles and operate machinery:
Enap-NL does not affect driving or operating machinery, however, some patients (mainly at the beginning of treatment) may experience arterial hypotension and dizziness, which contribute to a decrease in the ability to drive a car and operate machinery.
Therefore, at the beginning of treatment, it is recommended to avoid driving, operating machinery, and performing other work that requires concentration until a response to treatment has been established.
Active ingredient
Active ingredient
Hydrochlorothiazide, Enalapril
Composition
Composition
1 tablet contains:
Active ingredients:
Enalapril maleate 20 mg.
Hydrochlorothiazide 12.5 mg.
Excipients:
sodium bicarbonate,
lactose monohydrate,
calcium hydrogen phosphate (anhydrous),
corn starch,
talc,
magnesium stearate.
Pregnancy
Pregnancy
Enap-HL is contraindicated during pregnancy, breastfeeding and under the age of 14 years.
Contraindications
Contraindications
Hypersensitivity to the components of the drug.
Hypersensitivity to sulfonamides.
Anuria.
Severe renal dysfunction.
Hereditary or idiopathic angioedema.
Angioedema associated with the use of ACE inhibitors.
Primary hyperaldosteronism.
Addison’s disease.
Porphyria.
Children and teenagers up to 18 years of age.
With caution:
Bilateral renal artery stenosis.
Stenosis of the artery of a single kidney.
Renal dysfunction.
Severe stenosis of the aortic mouth.
Idiopathic hypertrophic subaortic stenosis.
IHD.
Cerebrovascular diseases.
Chronic heart failure.
Severe autoimmune systemic connective tissue diseases.
Inhibition of bone marrow hematopoiesis.
Diabetes mellitus.
Hyperkalemia.
Condition after kidney transplantation.
Severe liver and/or kidney dysfunction.
Conditions accompanied by a decrease in blood volume:
As a result of diuretic therapy.
When limiting the consumption of table salt.
Diarrhea.
Vomit .
Gout.
Elderly patients.
Side Effects
Side Effects
nausea, vomiting, abdominal discomfort;
dizziness when suddenly rising from a lying position, headache, dizziness;
dry cough;
allergic reactions.
From the cardiovascular system:
Palpitations, various cardiac arrhythmias, marked decrease in blood pressure, Orthostatic hypotension, cardiac arrest, myocardial infarction, cerebrovascular stroke, angina pectoris, Raynaud’s syndrome, necrotizing angiitis.
From the digestive system:
Dry mouth, glossitis, stomatitis, inflammation of the salivary glands, Anorexia, nausea, vomiting, diarrhea, constipation, flatulence, epigastric pain, intestinal colic, ileus, pancreatitis, liver failure, hepatitis, jaundice, melena.
From the respiratory system:
Rhinitis, sinusitis, pharyngitis, hoarseness, bronchospasm, asthma, pneumonia, pulmonary infiltrates, eosinophilic pneumonia, pulmonary embolism, pulmonary infarction, non-productive “dry” cough, respiratory distress syndrome, including pneumonitis and pulmonary edema.
From the central nervous system and peripheral nervous system:
Depression, ataxia, drowsiness, insomnia, anxiety, nervousness, peripheral neuropathy (Paresthesia, dysesthesia).
From the urinary system:
Oliguria, renal failure, renal dysfunction, interstitial nephritis.
From the reproductive system:
Gynecomastia, decreased potency.
From the senses:
Visual impairment, impaired taste, impaired sense of smell, tinnitus, conjunctivitis, dry conjunctiva, lacrimation.
From the hematopoietic system:
Leukocytosis, eosinophilia, neutropenia, leukopenia, agranulocytosis, anemia, hypoglobinemia, pancytopenia.
From the side of metabolism:
Hypokalemia, hyperkalemia, hypomagnesemia, hypercalcemia, hyponatremia, hypochloremic alkalosis, hyperglycemia, glycosuria, hyperuricemia, hypercholesterolemia, hypertriglyceridemia, increased activity of liver enzymes, hyperbillirubinemia.
Dermatological reactions:
Sweating, rash, shingles, alopecia.
Allergic reactions:
Urticaria, itching, skin rash, exfoliative dermatitis, toxic epidermal necrolysis, erythema multiforme, Steven-Johnson syndrome, photosensitivity, hypersensitivity reactions (angioedema, thrombocytopenic purpura), anaphylactic reactions.
Others:
Weakness, fever, lupus-like syndrome described in the literature (fever, myalgia and arthralgia, serositis, vasculitis, skin rash, increased ESR, leukocytosis, eosinophilia, positive test for antinuclear antibodies).
Interaction
Interaction
The simultaneous use of Enap-NL with other antihypertensive drugs, barbiturates, tricyclic antidepressants, phenothiazines and narcotic drugs, as well as with ethanol, enhances the antihypertensive effect of Enap-NL.
Analgesics and NSAIDs, a large amount of salt in the diet, simultaneous intake of cholestyramine or colestipol reduce the effect of Enap-NL.
If possible, the simultaneous use of Enap-NL and lithium preparations should be avoided, since lithium intoxication may develop due to decreased excretion of lithium. It is necessary to control the concentration of lithium in the blood serum; its dose is adjusted accordingly.
The simultaneous use of Enap-NL and NSAIDs, analgesics (due to inhibition of prostaglandin synthesis) may reduce the effectiveness of enalapril and increase the risk of deterioration of renal function and/or heart failure. In some patients, concomitant treatment may also reduce the antihypertensive effect of enalapril, so patients should be closely monitored.
The simultaneous use of Enap-NL with potassium-sparing diuretics (including spironolactone, amiloride, triamterene) or the addition of potassium can lead to hyperkalemia.
The simultaneous use of Enap-NL with allopurinol, cytostatics, immunosuppressants or systemic corticosteroids can cause leukopenia, anemia or pancytopenia, therefore periodic monitoring of the hemogram is required.
Acute renal failure was reported in 2 patients after kidney transplantation who were simultaneously receiving enalapril and cyclosporine. It is believed that acute renal failure resulted from the reduction in renal blood flow caused by cyclosporine and the reduction in glomerular filtration caused by enalapril. Therefore, caution is necessary when using enalapril and cyclosporine simultaneously.
The simultaneous use of Enap-NL with sulfonamides and oral hypoglycemic agents from the sulfonylurea group may cause hypersensitivity reactions (cross-hypersensitivity is possible).
Caution is required when using Enap-NL simultaneously with cardiac glycosides. Possible hydrochlorothiazide-induced hypovolemia, hypokalemia and hypomagnesemia may increase the toxicity of glycosides.
The simultaneous use of Enap-NL with GCS increases the risk of hypokalemia.
With the simultaneous use of Enap-NL and theophylline, enalapril can reduce T1/2 of theophylline.
With the simultaneous use of Enap-NL and cimetidine, T1/2 of enalapril may increase.
The risk of hypotension increases during general anesthesia or the use of non-depolarizing muscle relaxants (eg, tubocurarine).
Overdose
Overdose
If the patient takes too many tablets at once, you should immediately call a doctor.
Symptoms: increased diuresis, marked decrease in blood pressure with bradycardia or other heart rhythm disturbances, convulsions, paresis, paralytic ileus, disturbances of consciousness (including Coma), renal failure, impaired blood pressure, blood electrolyte imbalance.
Treatment: the patient is transferred to a horizontal position with a low headboard. In mild cases, gastric lavage and ingestion of saline solution are indicated. In more serious cases, measures aimed at stabilizing blood pressure are indicated: intravenous administration of saline, plasma substitutes.
It is necessary to monitor the level of blood pressure, heart rate, respiratory rate, serum concentration of urea, creatinine, electrolytes and diuresis of the patient. If necessary, intravenous administration of angiotensin II, hemodialysis (enalaprilat excretion rate is 62 ml/min).
Storage conditions
Storage conditions
At a temperature not exceeding 30 °C
Shelf life
Shelf life
5 years
Manufacturer
Manufacturer
KRKA dd Novo Mesto, Slovenia
Additional information
| Shelf life | 5 years |
|---|---|
| Conditions of storage | At a temperature not exceeding 30 °C |
| Manufacturer | KRKA dd Novo mesto, Slovenia |
| Medication form | pills |
| Brand | KRKA dd Novo mesto |
Other forms…
Related products
Buy Enap-NL 20, tablets 12.5mg+20 mg 20 pcs with delivery to USA, UK, Europe and over 120 other countries.