No products in the cart.
Enalapril, tablets 10 mg 20 pcs
€1.00
Out of stock
(E-mail when Stock is available)
Description
Enalapril is an antihypertensive drug from the ACE inhibitor group. Enalapril is a “prodrug”: as a result of its hydrolysis enalaprilat is formed, which inhibits ACE. Its mechanism of action is related to the reduction of angiotensin I and angiotensin II, the reduction of which leads to a direct reduction of aldosterone secretion. This reduces total peripheral vascular resistance, systolic and diastolic blood pressure (BP), post- and preload on the myocardium. It dilates arteries to a greater extent than veins, and there is no reflex increase in heart rate.
Hypotensive effect is more pronounced at high plasma renin levels than at normal or reduced levels. Decrease in BP within therapeutic limits does not affect the cerebral blood flow, blood flow in the brain vessels is maintained at a sufficient level even against the background of reduced blood pressure. It increases coronary and renal blood flow.
Long-term use reduces myocardial hypertrophy of the left ventricle and myocytes of the walls of the resistive arteries, prevents the progression of heart failure and slows the development of dilatation of the left ventricle.
Improves blood supply to ischemic myocardium. Reduces platelet aggregation. Has some diuretic effect. Time of onset of hypotensive effect when taken orally -1 hour reaches a maximum after 4-6 hours and lasts up to 24 hours. In some patients, therapy for several weeks is necessary to achieve optimal blood pressure levels.
With heart failure noticeable clinical effect is observed with long-term use – 6 months or more.
Indications
Indications
Arterial hypertension (symptomatic, renovascular, including with scleroderma, etc.), CHF I-III stages; prevention of coronary ischemia in patients with LV dysfunction, asymptomatic LV dysfunction.
Pharmacological effect
Pharmacological effect
Enalapril is an antihypertensive drug from the group of ACE inhibitors. Enalapril is a “prodrug”: as a result of its hydrolysis, enalaprilat is formed, which inhibits ACE. The mechanism of its action is associated with a decrease in the formation of angiotensin II from angiotensin I, a decrease in the content of which leads to a direct decrease in the release of aldosterone. At the same time, total peripheral vascular resistance, systolic and diastolic blood pressure (BP), post- and preload on the myocardium decrease. It dilates arteries to a greater extent than veins, while there is no reflex increase in heart rate.
The hypotensive effect is more pronounced with high plasma renin levels than with normal or reduced levels. A decrease in blood pressure within therapeutic limits does not affect cerebral circulation; blood flow in the vessels of the brain is maintained at a sufficient level even against the background of reduced blood pressure. Strengthens coronary and renal blood flow.
With long-term use, hypertrophy of the left ventricle of the myocardium and myocytes of the walls of resistive arteries decreases, prevents the progression of heart failure and slows down the development of left ventricular dilatation.
Improves blood supply to ischemic myocardium. Reduces platelet aggregation. Has some diuretic effect. The onset of the hypotensive effect when taken orally is 1 hour, reaches a maximum after 4-6 hours and lasts up to 24 hours. In some patients, therapy is necessary for several weeks to achieve an optimal level of blood pressure.
In heart failure, a noticeable clinical effect is observed with long-term use – 6 months or more.
Special instructions
Special instructions
Caution must be exercised when prescribing to patients with reduced blood volume (as a result of diuretic therapy, limiting salt intake, hemodialysis, diarrhea and vomiting) – the risk of a sudden and pronounced decrease in blood pressure is increased after using even the initial dose of an ACE inhibitor. Transient hypotension is not a contraindication for continuing treatment with the drug after stabilization of blood pressure. In case of repeated pronounced decrease in blood pressure, the dose should be reduced or the drug discontinued.
If an excessive decrease in blood pressure develops, the patient is transferred to a horizontal position with a low head, and, if necessary, a 0.9% NaCl solution and plasma-substituting drugs are administered.
The use of high-flow dialysis membranes (including AN69) increases the risk of developing an anaphylactic reaction. Correction of the dosage regimen on days free from dialysis should be carried out depending on blood pressure.
Before and during treatment with ACE inhibitors, it is necessary to monitor blood pressure, blood parameters (Hb, K+ concentration, creatinine, urea, liver enzyme activity), and protein in the urine.
Patients with decompensated CHF, ischemic heart disease and cerebral vascular diseases, in whom a sharp decrease in blood pressure can lead to myocardial infarction, stroke or impaired renal function, should be carefully monitored. Sudden cessation of treatment does not lead to withdrawal syndrome (a sharp rise in blood pressure).
Patients with a history of angioedema have an increased risk of developing it when taking ACE inhibitors.
For newborns and infants who have been exposed in utero to ACE inhibitors, it is recommended to conduct careful monitoring for timely detection of a pronounced decrease in blood pressure, oliguria, hyperkalemia and neurological disorders that may be due to a decrease in renal and cerebral blood flow with a decrease in blood pressure caused by ACE inhibitors. In case of oliguria, it is necessary to maintain blood pressure and renal perfusion by administering appropriate fluids and vasoconstrictor drugs.
In patients with reduced renal function, the single dose should be reduced or the intervals between doses should be increased.
Before studying the functions of the parathyroid glands, enalapril should be discontinued.
Caution should be exercised when performing physical exercise or in hot weather (risk of dehydration and excessive decrease in blood pressure due to a decrease in blood volume).
Before surgery (including dentistry), it is necessary to warn the surgeon/anesthesiologist about the use of ACE inhibitors.
During the treatment period, care must be taken when driving vehicles and engaging in other potentially hazardous activities that require increased concentration and speed of psychomotor reactions (dizziness is possible, especially after taking the initial dose of an ACE inhibitor in patients taking diuretic drugs).
Active ingredient
Active ingredient
Enalapril
Composition
Composition
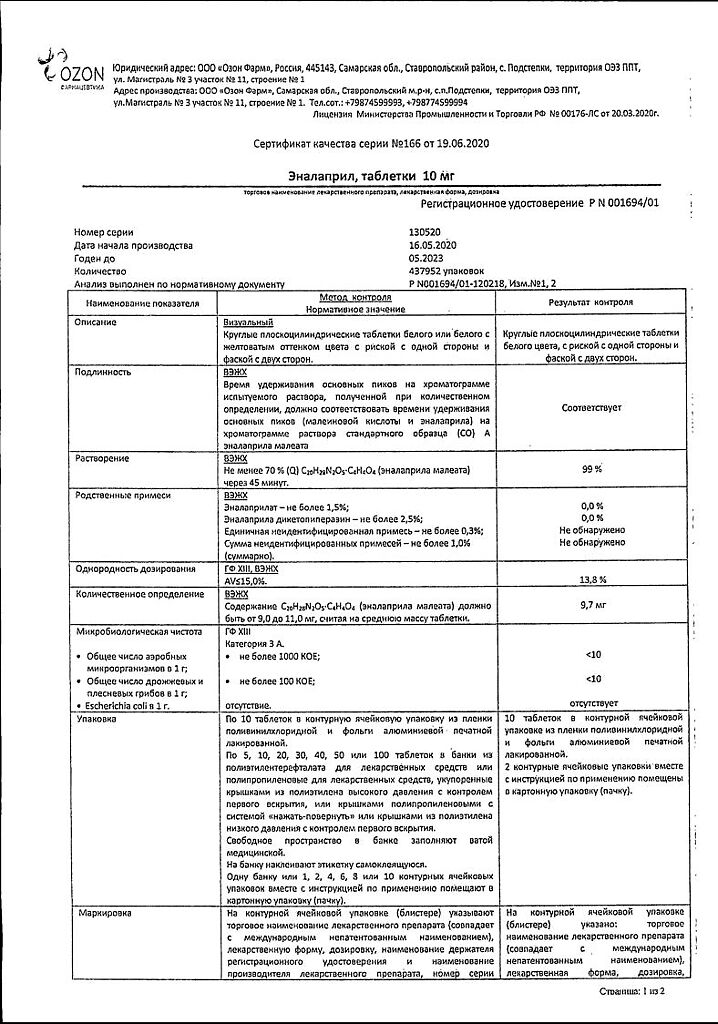
1 tablet contains: enalapril maleate – 10 mg.
Excipients: lactose monohydrate, magnesium carbonate, gelatin, crospovidone, magnesium stearate.
Contraindications
Contraindications
Hypersensitivity to enalapril or other ACE inhibitors, pregnancy, lactation.
With caution. History of angioedema during therapy with ACE inhibitors, hereditary or idiopathic angioedema, aortic stenosis, cerebrovascular diseases (including cerebrovascular insufficiency), coronary artery disease, coronary insufficiency, severe autoimmune systemic connective tissue diseases (including SLE, scleroderma), suppression of bone marrow hematopoiesis, diabetes diabetes, hyperkalemia, bilateral renal artery stenosis, artery stenosis of a single kidney, condition after kidney transplantation, renal and/or liver failure, Na+-restricted diet, conditions accompanied by a decrease in blood volume (including diarrhea, vomiting), elderly age, age under 18 years (safety and effectiveness of use have not been studied).
Side Effects
Side Effects
From the cardiovascular system: excessive decrease in blood pressure, orthostatic collapse, rarely – chest pain, angina pectoris, myocardial infarction (usually associated with a pronounced decrease in blood pressure), arrhythmias (atrial brady- or tachycardia, atrial fibrillation), palpitations, thromboembolism of the branches of the pulmonary artery.
From the nervous system: dizziness, fainting, headache, weakness, insomnia, anxiety, confusion, fatigue, drowsiness (2-3%), very rarely when used in high doses – nervousness, depression, paresthesia.
From the senses: disorders of the vestibular apparatus, hearing and vision impairment, tinnitus.
From the digestive system: dryness of the oral mucosa, loss of appetite, dyspeptic disorders (nausea, diarrhea or constipation, vomiting, abdominal pain), intestinal obstruction, pancreatitis, impaired liver function and biliary excretion, hepatitis, jaundice.
From the respiratory system: non-productive “dry” cough, interstitial pneumonitis, bronchospasm, shortness of breath, rhinorrhea, pharyngitis.
Allergic reactions: skin rash, angioedema of the face, small intestine (very rarely), limbs, lips, tongue, glottis and/or larynx, dysphonia, exfoliative dermatitis, exudative erythema multiforme (including Stevens-Johnson syndrome), toxic epidermal necrolysis (Lyell’s syndrome), pemphigus (pemphigus), cutaneous itching, urticaria, photosensitivity, serositis, vasculitis, myositis, arthralgia, arthritis, stomatitis, glossitis.
From laboratory parameters: hypercreatininemia, increased urea concentration, increased activity of “liver” transaminases, hyperbilirubinemia, hyperkalemia, hyponatremia, decreased Hb and hematocrit, increased ESR, thrombocytopenia, neutropenia, agranulocytosis (in patients with autoimmune diseases), eosinophilia.
From the urinary system: impaired renal function, proteinuria.
Other: alopecia, decreased libido, flushing of the face. Cases of hypoglycemia have been reported in patients with diabetes mellitus who took insulin and oral hypoglycemic drugs.
Interaction
Interaction
Enhances the effect of ethanol, slows down the excretion of Li+.
Weakens the effect of drugs containing theophylline.
The hypotensive effect is reduced by NSAIDs, incl. selective COX-2 inhibitors, estrogens; enhance – diuretics, other antihypertensive drugs (beta-blockers, methyldopa, nitrates, BMCA, hydralazine, prazosin), drugs for general anesthesia, ethanol.
Potassium-sparing diuretics and potassium-containing drugs increase the risk of developing hyperkalemia.
Drugs that cause bone marrow suppression increase the risk of developing neutropenia and/or agranulocytosis.
With the simultaneous use of ACE inhibitors and gold preparations for parenteral use (sodium aurothiomalate), a symptom complex has been described, including facial flushing, nausea, vomiting and decreased blood pressure.
Concomitant use with insulin and oral hypoglycemic drugs increases the risk of developing hypoglycemia.
Immunosuppressants, allopurinol, cytostatics increase hematotoxicity.
Overdose
Overdose
Symptoms: excessive decrease in blood pressure, up to the development of collapse, myocardial infarction, acute cerebrovascular accident or thromboembolic complications; convulsions, stupor.
Treatment: the patient is transferred to a horizontal position with a low headboard. In mild cases, gastric lavage and ingestion of saline solution are indicated; in more serious cases, measures aimed at stabilizing blood pressure are indicated: intravenous administration of a 0.9% NaCl solution, plasma substitutes, if necessary, intravenous administration of angiotensin II, hemodialysis (enalaprilat excretion rate – 62 ml/min).
Storage conditions
Storage conditions
In a dry place, protected from light, at a temperature not exceeding 25 °C
Shelf life
Shelf life
3 years
Manufacturer
Manufacturer
Ozon, Russia
Additional information
| Shelf life | 3 years |
|---|---|
| Conditions of storage | In a dry, light-protected place at a temperature not exceeding 25 °C |
| Manufacturer | Ozon, Russia |
| Medication form | pills |
| Brand | Ozon |
Other forms…
Related products
Buy Enalapril, tablets 10 mg 20 pcs with delivery to USA, UK, Europe and over 120 other countries.