No products in the cart.
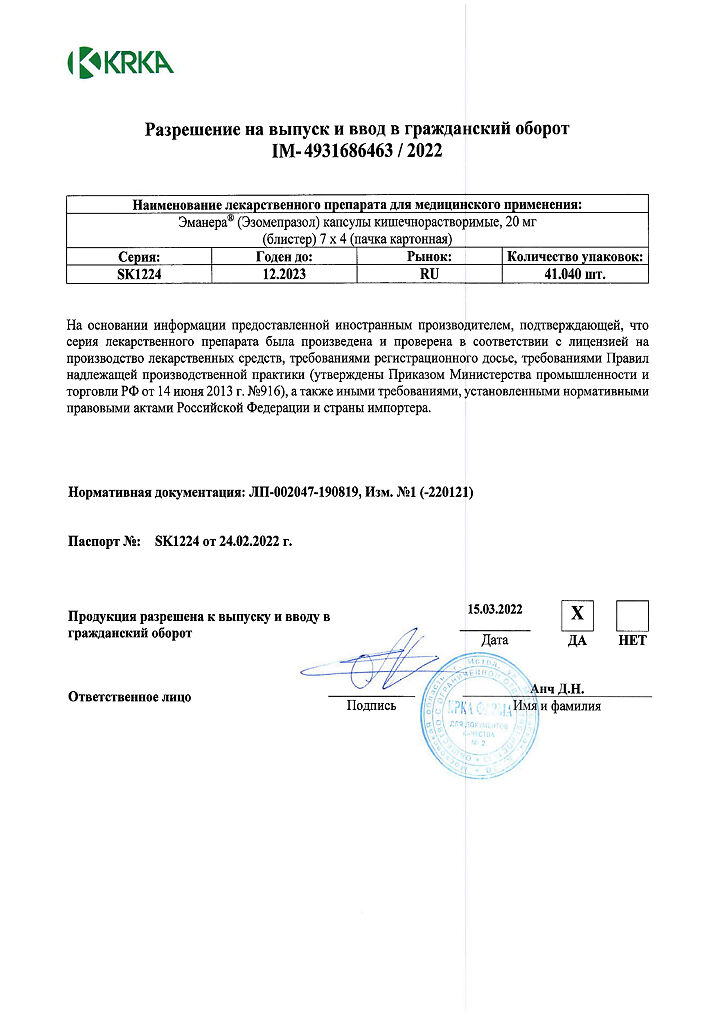
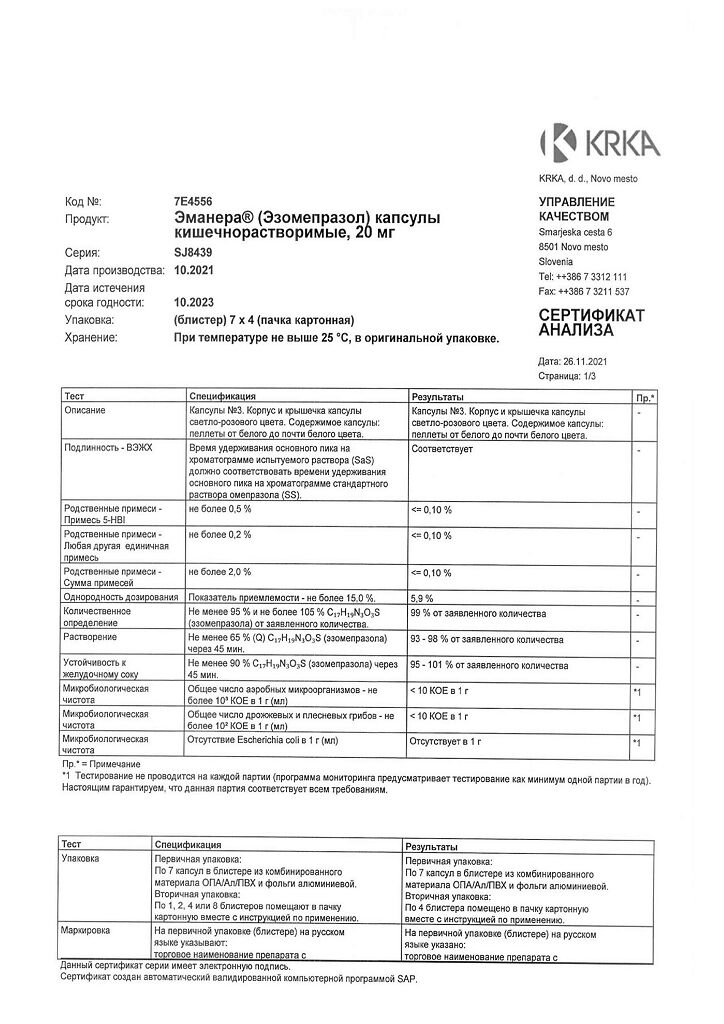
Emanera, 20 mg capsules 28 pcs
€6.64 €5.53
EAN: 3838989625610
SKU: 219885
Categories: Medicine, Stomach, intestines, liver, Ulcer and gastritis
Description
Gastric and 12 duodenal ulcer, Subscapular pain, Reflux esophagitis, Sour belching, GI infections caused by Helicobacter pylori, Heartburn
Gastroesophageal reflux disease (GERD):
– treatment of erosive reflux esophagitis;
– long-term maintenance treatment after healing of erosive reflux esophagitis to prevent relapse;
–-symptomatic treatment of GERD.
– Gastric and duodenal ulcer.
As part of the combined therapy:
– treatment of duodenal ulcer associated with Helicobacter pylori;
– prevention of recurrence of peptic ulcer associated with Helicobacter pylori.
– Prolonged acid-suppressive therapy in patients who have suffered bleeding from a peptic ulcer (after intravenous use of drugs that reduce gastric gland secretion to prevent recurrence).
– Patients taking long-term nonsteroidal anti-inflammatory drugs (NSAIDs):
– healing of gastric ulcers associated with taking NSAIDs;
– prevention of gastric and duodenal ulcers associated with taking NSAIDs in patients at risk.
– Zollinger-Ellison syndrome and other conditions characterized by abnormal hypersecretion of gastric glands, including idiopathic hypersecretion.
Indications
Indications
Gastroesophageal reflux disease (GERD):
– treatment of erosive reflux esophagitis;
-long-term maintenance treatment after healing of erosive reflux esophagitis to prevent relapse;
– symptomatic treatment of GERD.
· Peptic ulcer of the stomach and duodenum.
As part of combination therapy:
– treatment of duodenal ulcer associated with Helicobacter pylori;
– prevention of relapses of peptic ulcers associated with Helicobacter pylori.
· Long-term acid suppression therapy in patients who have suffered bleeding from a peptic ulcer (after intravenous use of drugs that reduce the secretion of gastric glands to prevent relapse).
· Patients taking non-steroidal anti-inflammatory drugs (NSAIDs) for a long time:
– healing of stomach ulcers associated with taking NSAIDs;
– prevention of gastric and duodenal ulcers associated with taking NSAIDs in patients at risk.
· Zollinger-Ellison syndrome and other conditions characterized by pathological hypersecretion of the gastric glands, including idiopathic hypersecretion.
Pharmacological effect
Pharmacological effect
gastric glands secretion reducing agent – proton pump inhibitor
Special instructions
Special instructions
Severe renal failure (experience is limited).
Contraindicated in children under 12 years of age (no data on efficacy and safety) and contraindicated in children over 12 years of age for indications other than gastroesophageal reflux disease (GERD).
Children over 12 years old
Gastroesophageal reflux disease (GERD)
Treatment of erosive reflux esophagitis: 40 mg once a day for 4 weeks.
Long-term maintenance treatment after healing of erosive reflux esophagitis to prevent relapse: 20 mg once a day.
Symptomatic treatment of GERD: 20 mg once daily for patients without esophagitis. After eliminating the symptoms, you can switch to the on-demand regimen of taking the drug, i.e. take Emanera® 20 mg once daily when symptoms return.
Renal failure: no dose adjustment of Emanera® is required. However, experience with the use of esomeprazole in patients with severe renal failure is limited; therefore, caution should be exercised when prescribing Emanera® to such patients (see section “Pharmacokinetics”).
Liver failure: In case of mild to moderate liver failure, no dose adjustment of Emanera® is required. For patients with severe liver failure, the maximum daily dose should not exceed 20 mg.
Elderly patients: no dose adjustment of Emanera® is required.
If alarming symptoms appear (for example, significant, spontaneous weight loss, repeated vomiting, dysphagia, vomiting with blood or melena), or if a gastric ulcer is suspected or detected, it is necessary to exclude a malignant neoplasm, since the use of Emanera® may reduce the severity of symptoms and delay the diagnosis.
Patients taking Emanera® for a long time (especially more than a year) should be under regular medical supervision.
Patients taking the drug “on demand” should be informed of the need to consult a doctor if the nature of symptoms changes. Given the fluctuations in the concentration of esomeprazole in the blood plasma when using the drug in the “on demand” mode, interactions with other drugs should be taken into account (see section “Interaction with drugs”).
When using esomeprazole for the eradication of Helicobacter pylori, possible interactions between the components of triple therapy should be taken into account. Clarithromycin is a potent inhibitor of CYP3A4, so contraindications and drug interactions with clarithromycin should be considered when prescribing triple therapy to patients concomitantly taking drugs metabolized by CYP3A4, such as cisapride.
Impact on laboratory results
An increase in the concentration of CgA in blood plasma may affect the results of studies conducted to diagnose neuroendocrine tumors. To avoid this effect, treatment with Emanera® should be discontinued at least 5 days before determining the concentration of CgA (see the “Pharmacodynamics” subsection of the “Pharmacological properties” section) in the blood plasma. If CgA and gastrin concentrations have not returned to the normal range after the initial measurement, a follow-up study should be performed 14 days after stopping PPI treatment.
Hypomagnesemia
Cases of severe hypomagnesemia have been reported in patients treated with PPIs such as esomeprazole for at least three months and, in most cases, for a year. Severe manifestations of hypomagnesemia, such as fatigue, tetany, delirium, seizures, dizziness, and ventricular arrhythmia, may occur but may develop gradually and may be missed. In patients with the most severe disorders, hypomagnesemia decreased after magnesium replacement therapy and PPI discontinuation.
In patients who are scheduled for long-term therapy or who are taking PPIs concomitantly with digoxin or drugs that cause hypomagnesemia (eg, diuretics), health care providers should consider monitoring plasma magnesium levels before initiating PPI treatment and periodically during treatment.
Fractures of the femoral neck, carpal bones and spine
PPIs, especially when used in high doses and over a long period of time (more than 1 year), may moderately increase the risk of hip, wrist, and spine fractures, primarily in older adults or those with other identified risk factors. Observational studies suggest that PPIs may increase the overall risk of fracture by 10% to 40%. Some degree of this increase may be due to other risk factors. Patients at risk of developing osteoporosis should be treated in accordance with current clinical guidelines and should consume adequate amounts of vitamin D and calcium.
Subacute cutaneous lupus erythematosus (SCLE)
PPI use is associated with extremely rare cases of PCLE. If pathological changes in the skin occur, especially in exposed areas, accompanied by arthralgia, the patient should immediately seek medical help. The physician should consider discontinuing Emanera®. PCLE due to prior PPI therapy may increase the risk of developing PCLE during subsequent therapy with other PPIs.
Special information on excipients
Emanera® contains sucrose, therefore its use is contraindicated in patients with hereditary fructose intolerance, glucose-galactose malabsorption syndrome or sucrase-isomaltase deficiency.
During treatment with the drug, caution should be exercised when performing potentially hazardous activities that require increased concentration and speed of psychomotor reactions.
Active ingredient
Active ingredient
Esomeprazole
Composition
Composition
1 enteric capsule 20 mg/40 mg contains:
Pellet core:
Active ingredient:
Esomeprazole magnesium dihydrate 21.688 mg/43.376 mg (equivalent to esomeprazole magnesium 20.645 mg/41.290 mg, equivalent to esomeprazole 20.000 mg/40.000 mg)
Excipients: granulated sugar [sucrose, starch syrup], povidone K30, sodium lauryl sulfate
Pellet shell:
Opadry II White 85F28751*, magnesium hydroxycarbonate (heavy magnesium carbonate), methacrylic acid and ethyl acrylate copolymer [1:1], dispersion 30%**, talc, macrogol-6000, titanium dioxide (E171), polysorbate-80
Gelatin capsule
Composition of empty gelatin capsules:
Capsule body:
Iron dye red oxide (E172), titanium dioxide (E171), gelatin
Capsule cap:
Iron dye red oxide (E172), titanium dioxide (E171), gelatin
*Opadray II White 85F28751 is a mixture of:
Polyvinyl alcohol, titanium dioxide (E171), macrogol-3000, talc
**Eudragit L30D dispersion contains, in addition to methacrylic acid, ethyl acrylate copolymer and water, also sodium lauryl sulfate (0.7% based on the solids in the dispersion) and polysorbate-80 (2.3% based on the solids in the dispersion) as emulsifiers.
Pregnancy
Pregnancy
There is insufficient data on the use of esomeprazole in pregnant women.
In epidemiological studies, no fetotoxic effects or fetal development disorders were identified during the use of the racemic mixture of omeprazole.
In studies with esomeprazole in animals, no direct or indirect negative effects on the development of the embryo or fetus were detected, nor were there any direct or indirect negative effects on the course of pregnancy, childbirth and the postnatal period of development of the newborn. Pregnant women should be prescribed the drug only when the expected benefit to the mother outweighs the possible risk to the fetus.
It is currently unknown whether esomeprazole is excreted in breast milk, therefore Emanera® should not be used during breastfeeding.
Contraindications
Contraindications
· Hypersensitivity to esomeprazole, substituted benzimidazoles or other components of the drug.
· Children under 12 years of age (no data on efficacy and safety) and children over 12 years of age for indications other than gastroesophageal reflux disease (GERD).
· Simultaneous use with atazanavir and nelfinavir (see section “Interaction with other drugs”).
· Hereditary fructose intolerance, glucose-galactose malabsorption syndrome or sucrase-isomaltase deficiency (Emanera® contains sucrose).
Side Effects
Side Effects
The following are side effects, independent of the dosage regimen of Emanera®, noted with the use of esomeprazole, both during clinical trials and in post-marketing studies.
Classification of the frequency of side effects recommended by the World Health Organization (WHO):
very common ≥ 1/10
often ≥ 1/100 to < 1/10
uncommon ≥ 1/1000 to < 1/100
rarely from ≥ 1/10000 to < 1/1000
very rare <1/10000
frequency unknown cannot be estimated from available data.
Within each group, adverse effects are presented in order of decreasing severity.
Blood and lymphatic system disorders:
rarely: leukopenia, thrombocytopenia;
very rare: agranulocytosis, pancytopenia.
Immune system disorders:
rarely: hypersensitivity reactions (for example, fever, angioedema, anaphylactic reaction/anaphylactic shock).
Metabolic and nutritional disorders:
uncommon: peripheral edema;
rarely: hyponatremia;
very rare: hypomagnesemia, hypocalcemia due to severe hypomagnesemia, hypokalemia due to hypomagnesemia.
Mental disorders:
uncommon: insomnia;
rarely: depression, agitation, confusion;
very rare: hallucinations, aggressive behavior.
Nervous system disorders:
often: headache;
uncommon: dizziness, paresthesia, drowsiness;
rare: change in taste.
Visual disorders:
rarely: blurred vision.
Disorders of the respiratory system, chest and mediastinal organs:
rarely: bronchospasm.
Digestive system disorders:
often: abdominal pain, constipation, diarrhea, flatulence, nausea/vomiting, glandular polyps of the fundus of the stomach (benign);
uncommon: dryness of the oral mucosa;
rarely: stomatitis, gastrointestinal candidiasis;
very rare: microscopic colitis (confirmed histologically).
Disorders of the liver and biliary tract:
uncommon: increased activity of liver enzymes in the blood plasma;
rarely: hepatitis (with or without jaundice);
very rare: liver failure, hepatic encephalopathy in patients with liver disease.
Disorders of the skin and subcutaneous tissues:
uncommon: dermatitis, skin rash, itching, urticaria;
rarely: alopecia, photosensitivity;
very rarely: erythema multiforme, Stevens-Johnson syndrome, toxic epidermal necrolysis;
frequency unknown: subacute cutaneous lupus erythematosus (SCLE).
Musculoskeletal and connective tissue disorders:
uncommon: fracture of the femoral neck, wrist bones or spine;
rarely: arthralgia, myalgia;
very rarely: muscle weakness.
Renal and urinary tract disorders:
very rare: interstitial nephritis, renal failure has been reported in some patients.
Disorders of the genital organs and breast:
very rare: gynecomastia.
General disorders and disorders at the injection site:
rarely: malaise, increased sweating.
Interaction
Interaction
Effect of esomeprazole on the pharmacokinetics of other drugs
Drugs whose absorption depends on pH levels
A decrease in the secretion of hydrochloric acid in the stomach during treatment with esomeprazole and other PPIs can lead to changes in the absorption of drugs, the absorption of which depends on the level of acidity of the environment. Like antacids and other drugs that reduce gastric acidity, esomeprazole may reduce the absorption of ketoconazole, itraconazole and erlotinib and increase the absorption of drugs such as digoxin.
Co-administration of omeprazole 20 mg once daily with digoxin increased the bioavailability of digoxin by 10% (bioavailability of digoxin increased by up to 30% in two out of ten patients).
Omeprazole is known to interact with some antiviral drugs. The mechanism and clinical significance of these interactions are not always known. A decrease in gastric acidity during omeprazole therapy may affect the absorption of antiviral drugs. Interaction at the level of the CYP2C19 isoenzyme is also possible. During therapy with omeprazole, there is a decrease in the serum concentrations of some antiviral drugs (atazanavir and nelfinavir). Therefore, simultaneous use is contraindicated. Concomitant use of omeprazole (40 mg once daily) with atazanavir 300 mg/ritonavir 100 mg in healthy volunteers is accompanied by a marked decrease in the bioavailability of atazanavir (AUC, Cmax and minimum plasma concentration [Cmin] decreased by approximately 75%). Increasing the atazanavir dose to 400 mg did not compensate for the effect of omeprazole on the bioavailability of atazanavir.
Concomitant use of omeprazole with saquinavir increases the concentration of saquinavir in the blood serum.
Given the similar pharmacokinetic and pharmacodynamic properties of omeprazole and esomeprazole, the simultaneous use of esomeprazole with antiviral drugs such as atazanavir and nelfinavir is contraindicated.
Drugs metabolized by the CYP2C19 isoenzyme
Esomeprazole inhibits the CYP2C19 isoenzyme, the main isoenzyme in the metabolism of esomeprazole. Thus, with simultaneous use of esomeprazole with drugs in the metabolism of which the CYP2C19 isoenzyme is involved, such as diazepam, citalopram, imipramine, clomipramine, phenytoin, etc., the concentration of these drugs in the blood plasma may increase and, accordingly, a reduction in their dose may be required.
This especially needs to be taken into account when prescribing Emanera® in the on-demand mode. Thus, when used simultaneously with 30 mg of esomeprazole, the clearance of diazepam (a substrate of the CYP2C19 isoenzyme) is reduced by 45%.
Concomitant use of esomeprazole at a dose of 40 mg leads to an increase in plasma phenytoin concentrations in patients with epilepsy by 13%. It is recommended to monitor phenytoin plasma concentrations when initiating esomeprazole therapy and when discontinuing it.
When using omeprazole at a dose of 40 mg, the Cmax and AUC of voriconazole (a substrate of the CYP2C19 isoenzyme) increases by 15% and 41%, respectively.
Coagulation time with simultaneous long-term use of warfarin and esomeprazole at a dose of 40 mg remains within acceptable limits. However, a few cases of clinically significant increases in the international normalized ratio (INR) have been reported. It is recommended to monitor the INR at the beginning and at the end of concomitant use of esomeprazole and warfarin or other coumarin derivatives.
The use of omeprazole at a dose of 40 mg led to an increase in Cmax and AUC of cilostazol by 18% and 26%, respectively, for one of the active metabolites of cilostazol the increase was 29% and 69%, respectively.
The simultaneous use of esomeprazole at a dose of 40 mg with cisapride leads to an increase in the pharmacokinetic parameters of cisapride in healthy volunteers: AUC by 32% and T1/2 by 31%, but Cmax does not change significantly. The slight prolongation of the QT interval on the ECG, which is observed with cisapride monotherapy, was not increased by the addition of esomeprazole.
In some patients, an increase in the concentration of methotrexate in the blood serum was noted during simultaneous use with PPIs. When using high doses of methotrexate, the possibility of temporary withdrawal of esomeprazole should be considered.
Esomeprazole does not cause clinically significant changes in the pharmacokinetics of amoxicillin and quinidine.
The simultaneous short-term use of esomeprazole and naproxen or rofecoxib did not reveal a clinically significant pharmacokinetic interaction.
Results obtained from studies in healthy volunteers have demonstrated a pharmacokinetic/pharmacodynamic interaction between clopidogrel (loading dose 300 mg/maintenance dose 75 mg daily) and esomeprazole (40 mg daily orally), which results in a reduction in systemic exposure of the active metabolite of clopidogrel by an average of 40% and a decrease in maximum suppression (induced ADP) platelet aggregation by an average of 14%.
When clopidogrel was used simultaneously with a fixed combination of 20 mg esomeprazole/81 mg acetylsalicylic acid (ASA), compared with the use of clopidogrel alone, in a study involving healthy volunteers, a decrease in systemic exposure to the active metabolite of clopidogrel was observed by almost 40%. However, the maximum levels of inhibition of (ADP-induced) platelet aggregation in these healthy volunteers were similar in the clopidogrel and clopidogrel fixed combination (esomeprazole/ASA) groups.
Both observational and clinical studies have provided conflicting data regarding the clinical consequences of the pharmacokinetic/pharmacodynamic interaction of esomeprazole on major cardiovascular events. As a precaution, concomitant use of clopidogrel should be avoided.
When used concomitantly with tacrolimus, an increase in serum concentrations of tacrolimus is possible.
Effect of drugs on the pharmacokinetics of esomeprazole
The isoenzymes CYP2C19 and CYP3A4 are involved in the metabolism of esomeprazole.
With simultaneous use of esomeprazole with clarithromycin (500 mg 2 times a day) (inhibitor of the CYP3A4 isoenzyme), the AUC value of esomeprazole increases by 2 times.
Concomitant use of esomeprazole and a combined inhibitor of CYP2C19 and CYP3A4 isoenzymes (for example, voriconazole) may be accompanied by an increase in the AUC of esomeprazole by more than 2 times. Usually in such situations no change in the dose of esomeprazole is required. In patients with severely impaired liver function or if long-term therapy is necessary, the issue of reducing the dose of esomeprazole should be considered.
Drugs that induce the isoenzymes CYP2C19 and CYP3A4, such as rifampicin and St. John’s wort preparations, when used simultaneously with esomeprazole, may lead to a decrease in the concentration of esomeprazole in the blood plasma by accelerating the metabolism of esomeprazole.
Overdose
Overdose
To date, extremely rare cases of intentional overdose of esomeprazole have been described. Taking esomeprazole orally at a dose of 280 mg was accompanied by general weakness and symptoms from the gastrointestinal tract. A single dose of 80 mg of esomeprazole did not cause any negative effects. There is no known antidote for esomeprazole. Esomeprazole binds well to plasma proteins, so dialysis is ineffective. In case of overdose, symptomatic and general supportive treatment should be provided.
Clinical pharmacology
Clinical pharmacology
Pharmacodynamics
Esomeprazole is the S-isomer of omeprazole and suppresses the secretion of hydrochloric acid in the stomach due to a specific and targeted mechanism of action. Specifically inhibits the proton pump of parietal cells. Both isomers of omeprazole, R- and S-, have similar pharmacodynamic activities.
Mechanism of action
Esomeprazole is a weak base, so it accumulates and becomes active in the highly acidic environment of the secretory tubules of the parietal cells of the gastric mucosa, where it inhibits the activity of the H+/K+-ATPase enzyme. Suppresses both basal and stimulated secretion of hydrochloric acid.
Effect on acid secretion in the stomach
The effect develops within 1 hour after ingestion of 20 mg or 40 mg of esomeprazole. When repeated administration of 20 mg of esomeprazole once a day for 5 days, the average peak concentration of hydrochloric acid after stimulation with pentagastrin is reduced by 90% (on the 5th day of therapy, 6-7 hours after taking the drug).
In patients with gastroesophageal reflux disease (GERD) and the presence of clinical symptoms, after taking esomeprazole 20 mg or 40 mg daily for 5 days, gastric pH levels above 4.0 persisted for an average of 13 and 17 hours, respectively. The proportion of patients taking esomeprazole 20 mg/day whose gastric pH exceeded 4.0 for 8, 12 and 16 hours was 76%, 54% and 24%, respectively, and for esomeprazole 40 mg/day it was 97%, 92% and 56%.
The degree of suppression of acid secretion by esomeprazole is directly dependent on the area under the concentration-time curve.
Therapeutic effect achieved by suppressing acid secretion
Healing of reflux esophagitis when taking esomeprazole at a dose of 40 mg occurs in approximately 78% of patients after 4 weeks and in 93% of patients after 8 weeks of therapy.
Treatment with esomeprazole 20 mg twice daily for 1 week in combination with appropriate antibiotics leads to successful eradication of Helicobacter pylori in 90% of patients.
In case of uncomplicated peptic ulcer after eradication therapy (lasting from 7 to 10-14 days), continuation of monotherapy with antisecretory drugs is not required to heal the ulcer and eliminate symptoms.
Other effects associated with acid suppression
During treatment with antisecretory drugs, the serum concentration of gastrin increases. Due to a decrease in the secretion of hydrochloric acid, the concentration of chromogranin A (CgA) in the blood plasma increases. Increased concentrations of CgA in blood plasma may affect the results of examinations to detect neuroendocrine tumors. To prevent this effect, therapy with proton pump inhibitors (PPIs) must be suspended 5 days before testing plasma CgA concentrations. If after 5 days the concentrations of gastrin and CgA in the blood plasma have not returned to normal values, the study should be repeated 14 days after stopping the use of esomeprazole.
In some patients, after long-term therapy with esomeprazole, an increase in the number of enterochromaffin-like cells (ELC) was observed, probably associated with an increase in plasma gastrin concentrations.
With long-term use of antisecretory drugs, there was a slight increase in the incidence of gastric glandular cysts. These changes are due to physiological changes resulting from long-term suppression of acid secretion. Cysts are benign and reversible.
A decrease in the acidity of gastric contents while taking antisecretory drugs is accompanied by an increase in the content of microbial flora in the stomach, which is normally present in the gastrointestinal tract (GIT). PPI therapy may lead to a slight increase in the risk of developing gastrointestinal infections, such as those caused by Salmonella and Campylobacter spp.
Esomeprazole is more effective in healing gastric ulcers in patients treated with nonsteroidal anti-inflammatory drugs (NSAIDs), including selective COX-2 inhibitors, compared to ranitidine.
Esomeprazole has been shown to be highly effective in preventing gastric and duodenal ulcers in patients taking NSAIDs (for patients over 60 years of age and/or with a history of peptic ulcers), including selective cyclooxygenase-2 (COX-2) inhibitors.
Pharmacokinetics
Suction and distribution
Esomeprazole is unstable in an acidic environment, therefore it is taken orally in the form of enteric capsules containing drug pellets, the shell of which is also resistant to the action of gastric juice. Under invivo conditions, a small part of esomeprazole is converted to the R-isomer. Esomeprazole is rapidly absorbed, reaching maximum concentrations (Cmax) in the blood plasma approximately 1-2 hours after oral administration. Absolute bioavailability is 64% after a single dose of 40 mg, which increases to 89% with daily dosing of esomeprazole once daily. Bioavailability for esomeprazole at a dose of 20 mg is 50% and 68%, respectively. The volume of distribution at steady state in healthy volunteers is approximately 0.22 l/kg body weight. Connection with blood plasma proteins – 97%.
Eating slows down and reduces the absorption of esomeprazole, but has no significant clinical significance.
Metabolism and excretion
Esomeprazole is completely metabolized by the cytochrome P450 system in the liver. Most of it is metabolized with the participation of the polymorphic isoenzyme CYP2C19, which is responsible for the formation of hydroxy and demethylated metabolites. The rest of esomeprazole is metabolized by the CYP3A4 isoenzyme, which is responsible for the formation of esomeprazole sulfone, the main metabolite in the blood plasma.
The total plasma clearance after a single dose is approximately 17 l/hour and 9 l/hour after multiple doses. The half-life (T1/2) is 1.3 hours with long-term use of the drug once a day. The area under the concentration-time curve (AUC) increases with repeated dosing. The dose-dependent increase in AUC with repeated use is non-linear due to a decrease in first-pass metabolism through the liver, a decrease in clearance, probably caused by inhibition of the CYP2C19 isoenzyme by esomeprazole and/or its sulfonic metabolite. With a single daily dose, esomeprazole is completely eliminated from the blood plasma during the interval between doses. Esomeprazole does not accumulate.
The main metabolites of esomeprazole do not affect the secretion of hydrochloric acid in the stomach. Almost 80% of an oral dose of esomeprazole is excreted by the kidneys in the form of metabolites, and the rest is excreted through the intestines. Less than 1% of unchanged esomeprazole is found in urine.
Pharmacokinetics in selected patient groups
Approximately 2.9±1.5% of the population has reduced activity of the CYP2C19 isoenzyme. In such patients, esomeprazole is metabolized predominantly by the CYP3A4 isoenzyme. After multiple doses of esomeprazole 40 mg once daily, the mean AUC value is approximately 2 times higher than in patients with reduced CYP2C19 activity. The average Cmax values in blood plasma increase by approximately 60%.
In elderly patients (71-80 years), the metabolism of esomeprazole does not change significantly.
After a single dose of 40 mg esomeprazole, the average AUC value in women is approximately 30% higher than in men. Subsequently, with systematic daily dosing of esomeprazole once a day, no differences in pharmacokinetics were observed in patients of both sexes. These features do not affect the dose and method of administration of the drug.
The metabolism of esomeprazole may be impaired in patients with mild or moderate hepatic impairment. Metabolic rate is reduced in severe liver dysfunction, which is accompanied by a twofold increase in AUC. Therefore, the maximum daily dose of esomeprazole in these patients is 20 mg.
Pharmacokinetic studies have not been conducted in patients with reduced renal function. Since it is not esomeprazole itself that is excreted through the kidneys, but its metabolites, the metabolism of esomeprazole in these patients does not change.
After repeat dosing of 20 mg and 40 mg esomeprazole, AUC and time to maximum concentration (TCmax) were similar in children aged 12–18 years and adults.
Specifications
Specifications
If alarming symptoms appear (for example, significant spontaneous weight loss, repeated vomiting, dysphagia, vomiting with blood or melena), or if a gastric ulcer is suspected or detected, it is necessary to exclude a malignant neoplasm, since the use of Emanera may reduce the severity of symptoms and delay the diagnosis.
Patients taking Emanera for a long time (especially more than a year) should be under regular medical supervision.
Patients taking the drug on demand should be informed of the need to consult a doctor if the nature of symptoms changes.
Given the fluctuations in the concentration of esomeprazole in the blood plasma when using the drug in an on-demand regimen, interactions with other drugs should be taken into account (see “Interactions”). When using esomeprazole for the eradication of Helicobacter pylori, the possible interaction between the components of triple therapy should be taken into account. Clarithromycin is a potent inhibitor of CYP3A4, so contraindications and drug interactions with clarithromycin should be considered when prescribing triple therapy to patients concomitantly taking drugs metabolized by CYP3A4, such as cisapride.
Emanera contains sucrose, so its use is contraindicated in patients with hereditary fructose intolerance, glucose-galactose malabsorption syndrome or sucrase-isomaltase deficiency.
Impact on the ability to drive vehicles and other complex mechanisms. Emanera does not affect driving or operating other technical devices, which require increased concentration and speed of psychomotor reactions.
Storage conditions
Storage conditions
At a temperature not exceeding 25ºС, in original packaging.
Keep out of the reach of children.
Shelf life
Shelf life
2 years.
Do not use the drug after the expiration date.
Manufacturer
Manufacturer
KRKA dd Novo Mesto, Slovenia
Additional information
| Shelf life | 2 years. Do not use the product after the expiration date. |
|---|---|
| Conditions of storage | At temperature not exceeding 25ºC, in original packaging. Store out of reach of children. |
| Manufacturer | KRKA dd Novo mesto, Slovenia |
| Medication form | capsules |
| Brand | KRKA dd Novo mesto |
Related products
Buy Emanera, 20 mg capsules 28 pcs with delivery to USA, UK, Europe and over 120 other countries.