No products in the cart.
Eliquis, 2.5mg 20 pcs.
€25.70 €21.41
Description
Pharmacotherapeutic group
Direct acting anticoagulant – selective inhibitor of blood clotting factor Xa (FXa).
ATX code: B01AF02
Pharmacological properties
Pharmacodynamics
Apixaban is a potent direct FXa inhibitor that reversibly and selectively blocks the active center of the enzyme. The drug is intended for oral administration. Antithrombin III is not required for realization of antithrombotic activity of apixaban. Apixaban inhibits free and bound FXa. as well as prothrombinase activity. Apixaban has no direct direct effect on platelet aggregation, but indirectly inhibits platelet aggregation induced by thrombin. By inhibiting FXa activity, apixaban prevents thrombin and thrombus formation. As a result of FXa inhibition values of blood coagulation system are changed: prothrombin time, activated partial thromboplastin time (APTT) and international normalized ratio (INR) are prolonged. Changes in these parameters when using the drug in a therapeutic dose are insignificant and highly variable. Therefore, their use to assess the pharmacodynamic activity of apixaban is not recommended. In the thrombin generation test, apixaban decreased endogenous thrombin potential – a measure of thrombin formation in human plasma.
The inhibition of FXa activity by apixaban has been proven by a chromogenic test using Rotachrom heparin. The change in anti-FXa activity is directly proportional to an increase in plasma apixaban concentration, with maximum activity values observed when the maximum plasma apixaban concentration is reached. A linear relationship between the concentration and anti-FXa activity of apixaban is registered in a wide range of therapeutic doses of the drug. Changes in anti-FXa activity with changes in the dose and concentration of apixaban are more pronounced and less variable than blood clotting parameters.
Table 1 shows the estimated equilibrium concentrations and anti-FXa activity with apixaban for each indication. In patients receiving apixaban after elective hip or knee arthroplasty, the ratio of maximum to minimum anti-FXa activity between doses does not exceed 1.6. In patients receiving apixaban for prevention of stroke and systemic thromboembolism in non-valvular atrial fibrillation, the ratio is less than 1.7, and in patients receiving apixaban for treatment of deep vein thrombosis and prevention of recurrent deep vein thrombosis it is less than 2.2.
Table 1.
Estimated equilibrium concentrations (ng/mL) and anti-FXa activity (IU/mL)
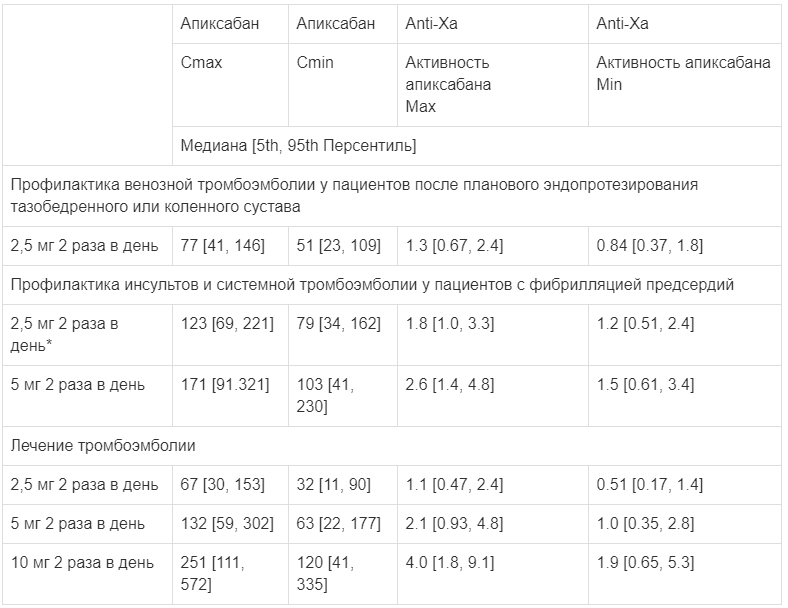

* Dose adjustment according to dose reduction criteria in the ARISTOTLE study.
Routine monitoring of apixaban’s anticoagulation effect is not required during therapy, but performing a calibrated quantitative test of anti-FXa activity may be useful in situations where information about the presence of apixaban in the blood may be useful in making decisions about continuation of therapy. Compared with warfarin, fewer bleeding events, including intracranial hemorrhage, have been reported with apixaban.
Pharmacokinetics
Eabsorption
The absolute bioavailability of apixaban is up to 50% when administered in doses up to 10 mg. Apixaban is rapidly absorbed from the gastrointestinal tract, its maximum concentration (Stakh) is reached within 3 – 4 hours after oral administration. Food intake has no effect on the values of area under the curve “concentration-time” (AUC) or Stakh apixaban. Apixaban pharmacokinetics for doses up to 10 mg is linear. When administering apixaban in doses above 25 mg, limitation of absorption of the drug is noted, which is accompanied by a decrease in its bioavailability. Metabolic parameters of apixaban are characterized by low to moderate inter- and intraindividual variability (the corresponding values of coefficient of variation are -20% and -30%, respectively).
After oral administration of apixaban at a dose of 10 mg as 2 crushed 5 mg tablets diluted in 30 ml of water, exposure to the drug was comparable to that after oral administration of 2 whole 5 mg apixaban tablets. After oral administration of apixaban at a dose of 10 mg as 2 crushed 5 mg tablets with 30 g apple puree, Stach and AUC values were 21% and 16% lower, respectively, than after administration of 2 whole 5 mg tablets.
After nasogastric tube administration of a crushed 5 mg tablet of apixaban diluted in 60 mL of 5% aqueous dextrose solution (5DV), apixaban exposure was comparable to that observed in other clinical studies involving healthy volunteers who received a single oral dose of 5 mg apixaban as a tablet.
Distribution
The binding of apixaban to human plasma proteins is approximately 87% and the volume of distribution (Vss) is approximately 21 liters.
Metabolism and excretion
About 25% of the dose taken is excreted as metabolites. The main route of excretion is through the intestine. Renal excretion of apixaban is approximately 27% of its total clearance.
The total clearance of apixaban is approximately 3.3 l/h, the half-life (T1/2) is about 12 h. O-demethylation and hydroxylation by 3-oxopiperidinyl residue are the main pathways of apixaban biotransformation. Apixaban is primarily metabolized with participation of CYP3A4/5 isoenzyme, to a lesser extent – CYP1A2, 2C8, 2C9, 2C19 and 2J2 isoenzymes. Unchanged apixaban is the main substance circulating in human plasma, there are no active metabolites circulating in the bloodstream. In addition, apixaban is a substrate of transport proteins, P-glycoprotein and breast cancer resistance protein (BCRP).
Kidney dysfunction
Kidney dysfunction has no effect on the maximum concentration of apixaban. However, there was an increase in apixaban concentrations correlated with the degree of renal function impairment as assessed by creatinine clearance values. In patients with mild (creatinine clearance – 51 ml/min to 80 ml/min), moderate (creatinine clearance – 30 ml/min to 50 ml/min) and severe (creatinine clearance – 15 ml/min to 29 ml/min) renal function disorders the AUC values of apixaban in plasma increased by 16%, 29% and 44%, respectively, compared to those patients with normal creatinine clearance values. At the same time, impaired renal function had no apparent effect on the relationship between plasma apixaban concentration and its anti-FXa activity.
There have been no studies of apixaban in patients with creatinine clearance less than 15 ml/min or on dialysis.
Hepatic impairment
There have been no studies of apixaban in severe hepatic insufficiency and active hepatobiliary pathology.
There were no significant changes in pharmacokinetic and pharmacodynamic parameters with single administration of 5 mg apixaban in patients with mild to moderate hepatic impairment (Child-Pugh grades A and B, respectively) compared to healthy volunteers. Changes in anti-FXa activity and INR in patients with mild to moderate hepatic impairment and healthy volunteers were comparable.
The use in elderly patients
The elderly patients (over 65 years) showed higher plasma concentrations of the drug than younger patients: the average AUC was approximately 32% higher.
Position of apixaban in women was 18% higher than in men.
Race and ethnicity
The results from the phase I studies suggest that there are no significant differences in apixaban pharmacokinetics between Caucasoid, Mongoloid, and Negroid races. The results of pharmacokinetic analyses in different populations performed as part of the apixaban clinical trial program, which included patients receiving apixaban after elective hip or knee arthroplasty, are generally consistent with the results of phase I studies.
Body weight
Patients with a body weight greater than 120 kg had plasma apixaban concentrations approximately 30% lower than patients with a body weight between 65 kg and 85 kg; patients with a body weight less than 50 kg had approximately 30% higher concentrations.
The relationship between pharmacokinetic and pharmacodynamic parameters
The relationship between pharmacokinetic and pharmacodynamic parameters (including anti-FXa activity. INR, prothrombin time. ACTV) of apixaban and its plasma concentrations have been studied for a wide range of drug doses (from 0.5 mg to 50 mg). It was shown that the relationship between apixaban concentration and FXa activity is best described using a linear model. Dependence of parameters of pharmacokinetics and pharmacodynamics of apixaban. evaluated in patients receiving apixaban in phase II and phase III clinical trials was consistent with that in healthy volunteers.
Indications
Indications
Prevention of venous thromboembolism in patients after planned hip or knee replacement.
Prevention of stroke and systemic thromboembolism in adult patients with non-valvular atrial fibrillation who have one or more risk factors (such as a history of stroke or transient ischemic attack, age 75 years or older, hypertension, diabetes mellitus, symptomatic chronic heart failure (NYHA functional class II or higher)). The exception is patients with severe and moderate mitral stenosis or artificial heart valves.
Treatment of deep vein thrombosis (DVT), pulmonary embolism (PE), as well as prevention of relapses of DVT and PE.
Pharmacological effect
Pharmacological effect
Pharmacotherapeutic group
Direct anticoagulant – selective inhibitor of blood coagulation factor Xa (FXa).
ATX code: B01AF02
Pharmacological properties
Pharmacodynamics
Apixaban is a potent direct FXa inhibitor that reversibly and selectively blocks the active site of the enzyme. The drug is intended for oral use. The antithrombotic activity of apixaban does not require the presence of antithrombin III. Apixaban inhibits free and bound FXa. as well as prothrombinase activity. Apixaban has no immediate direct effect on platelet aggregation, but indirectly inhibits thrombin-induced platelet aggregation. By inhibiting FXa activity, apixaban prevents the formation of thrombin and blood clots. As a result of suppression of FXa, the values of the blood coagulation system parameters change: prothrombin time, activated partial thromboplastin time (aPTT) are prolonged, and the international normalized ratio (INR) increases. Changes in these indicators when using the drug in a therapeutic dose are insignificant and highly variable. Therefore, their use to assess the pharmacodynamic activity of apixaban is not recommended. In the thrombin generation test, apixaban decreased the endogenous thrombin potential, an indicator of thrombin generation in human plasma.
The inhibition of FXa activity by apixaban has been demonstrated using the heparin Rotachrom chromogenic test. The change in anti-FXa activity is directly proportional to the increase in the concentration of apixaban in the blood plasma, with maximum activity values observed when the maximum concentration of apixaban in the blood plasma is reached. A linear relationship between the concentration and anti-FXa activity of apixaban is recorded over a wide range of therapeutic doses of the drug. Changes in anti-FXa activity with changes in apixaban dose and concentration are more pronounced and less variable than blood coagulation parameters.
Table 1 shows the estimated steady-state concentrations and anti-FXa activity for apixaban for each indication. In patients receiving apixaban after elective hip or knee replacement, the ratio of the maximum to minimum level of anti-FXa activity in the interval between doses of the drug does not exceed 1.6. In patients receiving apixaban for the prevention of stroke and systemic thromboembolism in non-valvular atrial fibrillation, this ratio is less than 1.7, and in patients receiving apixaban for the treatment of deep vein thrombosis and the prevention of recurrent deep vein thrombosis, it is less than 2.2.
Table 1.
Estimated steady-state concentrations (ng/ml) and anti-FXa activity (IU/ml)
*Dose adjustment according to dose reduction criteria in the ARISTOTLE study.
Routine monitoring of the anticoagulant effect of apixaban is not required during apixaban therapy, but a calibrated quantitative test of anti-FXa activity may be useful in situations where information about the presence of apixaban in the blood may be useful in deciding whether to continue therapy. Compared with warfarin, apixaban resulted in fewer bleeding events, including intracranial hemorrhage.
Pharmacokinetics
Suction
The absolute bioavailability of apixaban reaches 50% when used in doses of up to 10 mg. Apixaban is rapidly absorbed from the gastrointestinal tract, its maximum concentration (Cmax) is reached within 3 to 4 hours after oral administration. Food intake does not affect the area under the concentration-time curve (AUC) or Cmax of apixaban. The pharmacokinetics of apixaban for doses up to 10 mg is linear. When taking apixaban in doses above 25 mg, limited absorption of the drug is observed, which is accompanied by a decrease in its bioavailability. Apixaban metabolism rates are characterized by low to moderate inter- and intra-individual variability (the corresponding coefficient of variation values are -20% and -30%, respectively).
After oral administration of apixaban 10 mg as 2 crushed 5 mg tablets diluted in 30 ml of water, drug exposure was comparable to that after oral administration of 2 whole 5 mg apixaban tablets. After oral administration of apixaban 10 mg as 2 crushed 5 mg tablets with 30 g applesauce, Cmax and AUC values were 21% and 16% lower, respectively, than after administration of 2 whole 5 mg tablets.
Following nasogastric tube administration of a crushed 5 mg apixaban tablet diluted in 60 mL of 5% dextrose aqueous solution (5DW), apixaban exposure was comparable to that observed in other clinical studies in healthy volunteers receiving a single dose of apixaban 5 mg orally as a tablet.
Distribution
The binding of apixaban to human plasma proteins is approximately 87%, the volume of distribution (Vss) is approximately 21 L.
Metabolism and excretion
Approximately 25% of the dose taken is excreted as metabolites. The main route of elimination is through the intestines. Renal excretion of apixaban accounts for approximately 27% of its total clearance.
The total clearance of apixaban is approximately 3.3 l/h, the half-life (T1/2) is about 12 hours. O-demethylation and hydroxylation at the 3-oxopiperidinyl residue are the main pathways of biotransformation of apixaban. Apixaban is predominantly metabolized by the CYP3A4/5 isoenzyme, and to a lesser extent by the CYP1A2, 2C8, 2C9, 2C19 and 2J2 isoenzymes. Unchanged apixaban is the main substance circulating in human plasma; there are no active metabolites circulating in the bloodstream. In addition, apixaban is a substrate of the transport proteins P-glycoprotein and breast cancer resistance protein (BCRP).
Renal dysfunction
Impaired renal function does not affect the maximum concentration of apixaban. However, there was an increase in apixaban concentrations, which correlated with the degree of decrease in renal function, assessed by creatinine clearance values. In individuals with mild renal impairment (creatinine clearance – from 51 ml/min to 80 ml/min), moderate (creatinine clearance – from 30 ml/min to 50 ml/min) and severe (creatinine clearance – from 15 ml/min to 29 ml/min), the AUC values of apixaban in blood plasma increased by 16%, 29% and 44%, respectively, compared with individuals who had normal creatinine clearance values. However, renal dysfunction did not have an obvious effect on the relationship between the concentration of apixaban in the blood plasma and its anti-FXa activity.
Apixaban has not been studied in patients with creatinine clearance less than 15 mL/min or on dialysis.
Liver dysfunction
Apixaban has not been studied in severe liver failure and active hepatobiliary pathology.
There were no significant changes in pharmacokinetics and pharmacodynamics parameters with a single dose of apixaban 5 mg in patients with mild to moderate hepatic impairment (Child-Pugh classes A and B, respectively) compared with healthy volunteers. Changes in anti-FXa activity and INR were comparable in patients with mild to moderate hepatic impairment and healthy volunteers.
Use in elderly patients
Elderly patients (over 65 years of age) had higher plasma concentrations of the drug than younger patients: the mean AUC was approximately 32% higher.
Floor
Exposure to apixaban was 18% higher in women than in men.
Race and ethnicity
The results obtained in phase I studies indicate that there are no significant differences in the pharmacokinetics of apixaban between representatives of the Caucasian, Mongoloid and Negroid races. Results from cross-population pharmacokinetic analyzes performed in the apixaban clinical trial program, which included patients receiving apixaban after elective hip or knee replacement, were generally consistent with the results of the phase I studies.
Body weight
In patients weighing more than 120 kg, plasma concentrations of apixaban were approximately 30% lower than in patients weighing between 65 kg and 85 kg; in patients weighing less than 50 kg, this figure was approximately 30% higher.
Dependence of pharmacokinetics and pharmacodynamics parameters
The relationship between pharmacokinetics and pharmacodynamics parameters (including anti-FXa activity. INR), prothrombin time. APTT) of apixaban and its plasma concentrations have been studied for a wide range of drug doses (from 0.5 mg to 50 mg). The relationship between apixaban concentration and FXa activity has been shown to be best described using a linear model. Dependence of pharmacokinetics and pharmacodynamics parameters of apixaban. evaluated in patients treated with apixaban in phase II and III clinical studies was consistent with that in healthy volunteers.
Special instructions
Special instructions
Risk of bleeding
As with other anticoagulants, patients receiving Eliquis should be closely monitored for signs of bleeding. In conditions with a high risk of bleeding, the drug is recommended to be used with caution. The use of Eliquis® should be discontinued if severe bleeding develops (see sections “Side effects” and “Overdose”).
Although therapy with apixaban does not require continuous monitoring of its blood concentrations, it may sometimes be appropriate to perform a calibrated quantitative assay for anti-Factor Xa activity in those exceptional cases where data on the effects of apixaban can help guide clinical decisions, such as in overdose and emergency surgery (see section “Pharmacological properties”).
Interaction with other drugs affecting hemostasis
Due to the high risk of bleeding, simultaneous use with any other anticoagulants is contraindicated (see section “Contraindications”).
Concomitant use of Eliquis® with antiplatelet agents increases the risk of bleeding (see section “Interaction with other drugs”).
Therapy in patients concomitantly receiving NSAIDs, including ASA, should be carried out with caution.
After surgery, it is not recommended to use other platelet aggregation inhibitors simultaneously with Eliquis® (see section “Interaction with other drugs”).
In patients with atrial fibrillation and conditions requiring antiplatelet therapy with one or two drugs, a careful assessment of the potential benefits and risks should be performed before initiating concomitant therapy with Eliquis.
In a clinical trial in patients with atrial fibrillation, concomitant use of ASA resulted in an increased risk of major bleeding with both apixaban and warfarin. In this clinical study, combination therapy with two antiplatelet agents was rarely used.
The use of thrombolytic agents for the treatment of acute ischemic stroke
Experience with thrombolytic agents for the treatment of acute ischemic stroke in patients receiving apixaban is very limited.
Patients with artificial heart valves
The safety and effectiveness of the drug in patients with artificial heart valves with and without atrial fibrillation have not been studied. The use of Eliquis® is not recommended for this group of patients.
Surgical and invasive procedures
Eliquis should be discontinued at least 48 hours before elective surgery or an invasive procedure with a moderate or high risk of bleeding. This includes interventions for which the likelihood of clinically significant bleeding cannot be excluded or for which the risk of bleeding is unacceptable.
Eliquis should be discontinued at least 24 hours before elective surgery or an invasive procedure with a low risk of bleeding. This includes interventions where minimal, non-critical or easily controlled bleeding is expected.
If surgery or an invasive procedure cannot be delayed, it should be performed with appropriate caution, given the increased risk of bleeding. The risk of bleeding must be weighed against the need for emergency intervention. Nonvalvular atrial fibrillation usually does not require bridging therapy for 24 to 48 hours after stopping apixaban before surgery.
After an invasive procedure or surgery, Eliquis should be restarted as soon as possible, provided the clinical situation allows and sufficient hemostasis has been established (for information on cardioversion, see Dosage and Administration).
Temporary cessation of therapy
Discontinuation of anticoagulants, including Eliquis®, because of active bleeding, elective surgery, or an invasive procedure increases the patient’s risk of thrombosis. Interruptions in treatment should be avoided and if for any reason temporary cessation of anticoagulant therapy with Eliquis is required, it should be resumed as soon as possible.
Treatment of deep vein thrombosis and pulmonary embolism
It is not recommended to replace unfractionated heparin therapy with Eliquis® during the initiation of therapy in patients with PE with unstable hemodynamics, possible thrombolysis or pulmonary thrombectomy, since the safety and effectiveness of apixaban in these clinical situations have not been established.
Patients with cancer
The efficacy and safety of anixaban in the treatment of DVT, treatment of PE, and prevention of recurrent DVT and PE (rVTE) in patients with actively progressing cancer have not been established.
Patients with renal failure
Limited clinical data indicate that in patients with severe renal impairment (creatinine clearance 15-29 ml/min), plasma concentrations of anixaban are increased, which may increase the risk of bleeding. For the treatment of DVT, treatment of PE, and prevention of recurrent DVT and PE (rVTE), apixaban should be used with caution in patients with severe renal impairment (creatinine clearance 15-29 ml/min) (see Dosage and Administration and Pharmacological Properties). For the prevention of stroke and systemic embolism in patients with UAF, patients with severe renal impairment (creatinine clearance 15-29 ml/min) and patients with serum creatinine levels ≥ 1.5 mg/dL (133 μmol/L) in combination with age ≥ 80 years or body weight ≤ 60 kg, the dose of apixaban should be reduced to 2.5 mg twice a day (see section “Dosage and Administration”).
Due to the lack of clinical experience with apixaban in patients with creatinine clearance <15 ml/min or on dialysis, the use of apixaban in this group of patients is not recommended (see sections “Dosage and Administration” and “Pharmacological Properties”).
Elderly patients
With age, the risk of bleeding may increase (see section “Pharmacological properties”).
Also, the simultaneous use of Eliquis® and ASA in elderly patients requires caution due to a potentially higher risk of bleeding.
Body weight
With low body weight (< 60 kg), the risk of bleeding may increase (see section "Pharmacological properties").
Patients with liver failure
Eliquis is contraindicated in patients with liver disease associated with coagulopathy and a clinically significant risk of bleeding (see section “Contraindications”).
The drug is not recommended for patients with severe liver dysfunction (see section “Pharmacological properties”).
The drug should be used with caution in patients with mild to moderate liver dysfunction (stage A or B according to the Child-Pugh classification) (see sections “Dosage and Administration” and “Pharmacological Properties”).
Patients with elevated liver enzyme levels ALT/AST > 2 x ULN or total bilirubin ≥ 1.5 * ULN were excluded from clinical studies. Therefore, Eliquis® should be used with caution in this population (see section “Pharmacological properties”). Before starting to use Eliquis®, it is necessary to check biochemical indicators of liver function.
Interaction with cytochrome P450 inhibitors and P-gp inhibitors
The use of Eliquis is not recommended in patients receiving concomitant systemic treatment with potent inhibitors of CYP3A4 and P-glycoprotein, such as azole antimycotics (for example, ketoconazole, itraconazole, voriconazole and nosaconazole) and HIV protease inhibitors (for example, ritonavir). These drugs may increase apixaban exposure by 2-fold (see Drug Interactions section) or greater in the presence of additional factors that also increase apixaban exposure (eg, severe renal impairment).
Interaction with inducers of the isoenzyme CYP3A4 and P-gp
Concomitant use of Eliquis with potent inducers of the CYP3A4 isoenzyme and P-gp inducers (for example, rifampicin, phenytoin, carbamazepine, phenobarbital and St. John’s wort) may also lead to a decrease in the concentration of apixaban in the blood plasma (by approximately 50%). In a clinical study involving patients with atrial fibrillation, a decrease in efficacy and an increased risk of bleeding was noted with simultaneous use of apixaban and strong inducers of the CYP3A4 and P-gp isoenzymes compared with the use of apixaban alone.
The following recommendations apply to patients receiving systemic therapy with potent inhibitors of the isoenzyme CYP3A4 and P-gp (see section “Interaction with other drugs”):
– for the purpose of preventing venous thromboembolism in patients after planned hip or knee replacement, preventing stroke and systemic thromboembolism in non-valvular atrial fibrillation, as well as for preventing relapses of DVT and PE, apixaban should be used with caution:
– for the treatment of DVT and PE, apixaban should not be used as the effectiveness may be reduced.
Surgical interventions associated with femoral neck fracture
The efficacy and safety of apixaban have not been evaluated in clinical trials in patients undergoing surgery for hip fracture. Therefore, it is not recommended for these patients.
Laboratory parameters
The effect of the mechanism of action of apixaban on blood coagulation parameters (eg, prothrombin time (PT), INR, and activated partial thromboplastin time (aPTT)) was as expected. The observed changes in these blood clotting parameters at the intended therapeutic dose were small and had significant variability (see section “Pharmacological properties”).
Information on excipients
Eliquis® contains lactose. Patients with rare hereditary disorders such as galactose intolerance, Lapp lactase deficiency or glucose-galactose malabsorption should not take Eliquis.
Performing spinal, epidural anesthesia or punctures in patients receiving Eliquis®
When performing spinal or epidural anesthesia or diagnostic puncture of these areas in patients receiving antithrombotic drugs to prevent thromboembolism, there is a risk of developing epidural or spinal hematomas, which, in turn, can cause persistent or irreversible paralysis. This risk may further increase when using an installed epidural catheter in the postoperative period or when concurrently using other drugs that affect hemostasis. Established epidural or subarachnoid catheters should be removed at least 5 hours before the first dose of Eliquis. The risk may also increase with traumatic or repeated epidural or spinal punctures. Patients should be frequently monitored for signs and symptoms of neurological deficits (eg, numbness or weakness in the legs, bowel or bladder dysfunction). If a neurological abnormality is detected, urgent diagnosis and treatment is necessary. Before neuraxial intervention, the clinician should consider the potential benefits and risks for patients who are receiving or will receive anticoagulant therapy for thrombosis prophylaxis.
There is no clinical experience with the use of apixaban in patients with an installed intrathecal or epidural catheter. If this situation is necessary, based on the pharmacokinetic properties of apixaban, an interval of 20-30 hours (i.e., 2 half-lives) should be maintained between the last dose of apixaban taken and catheter removal, thus at least one dose of apixaban should be omitted before catheter removal. The next dose of apixaban can be used no earlier than 5 hours after removing the catheter. As with all new anticoagulant drugs, experience with apixaban in neuraxial blockade is limited and extreme caution should be exercised in this situation.
Impact on the ability to drive vehicles and machinery
Eliquis® does not affect or only slightly affects the ability to drive vehicles and operate machinery.
Active ingredient
Active ingredient
Apixaban
Composition
Composition
1 film-coated tablet contains:
Active substance: apixaban 2.5 mg.
Excipients: Lactose 50.25 mg, microcrystalline cellulose 41.00 mg, croscarmellose sodium 4.00 mg, sodium lauryl sulfate 1.00 mg, magnesium stearate 1.25 mg.
Film coating: Oadray II Yellow 4.0 mg (hypromellose 15 cps 1.48 mg, lactose monohydrate 1.24 mg, titanium dioxide 0.8 mg, triacetin 0.32 mg, iron dye yellow oxide 0.16 mg).
Pregnancy
Pregnancy
Use during pregnancy
There are no data on the use of apixaban in pregnant women. During preclinical studies, no direct or indirect negative effects on reproductive function were detected. The use of apixaban during pregnancy is contraindicated.
Use during breastfeeding
There is no information on the excretion of apixaban or its metabolites into breast milk in humans. Based on available data from animal studies, apixaban is excreted into breast milk. In studies in rats, drug concentrations in breast milk were many times higher than those in plasma (Cmax approximately 8 times higher. AUC approximately 30 times higher), which may indicate active transport of the drug into breast milk. A risk to breastfed infants cannot be excluded. If it is necessary to use Eliquis®, a decision should be made to stop taking the drug or stop breastfeeding.
Effect on fertility
Apixaban did not affect fertility in animal studies.
Contraindications
Contraindications
Hypersensitivity to apixaban or any other component of the drug.
Active clinically significant bleeding.
Liver diseases accompanied by disturbances in the blood coagulation system and a clinically significant risk of bleeding.
Diseases or conditions characterized by a significant risk of major bleeding: current or recent exacerbation of gastrointestinal ulcer; the presence of a malignant neoplasm with a high risk of bleeding; recent brain or spinal cord injury; recent surgery on the brain or spinal cord, as well as on the organ of vision; recent hemorrhagic stroke; established or suspected varicose veins of the esophagus; arteriovenous malformation; vascular aneurysm or pronounced intraspinal or intracerebral vascular changes.
Impaired renal function with creatinine clearance less than 15 ml/min, as well as use in patients on dialysis.
Age up to 18 years (data on the use of the drug is not available).
Pregnancy (there are no data on the use of the drug).
Breastfeeding period (data on the use of the drug are not available).
Concomitant use with any other anticoagulant drugs, including unfractionated heparin (UFH), low molecular weight heparins (LMWH) (enoxaparin, dalteparin, etc.), heparin derivatives (fondaparinux, etc.), oral anticoagulants (warfarin, rivaroxaban, dabigatran, etc.), except in situations where the patient is transferred to therapy or with apixaban therapy or if unfractionated heparin is prescribed in doses necessary to maintain the patency of a central venous or arterial catheter (see section “Interaction with other medicinal products”),
Congenital lactase deficiency, lactose intolerance, glucose-galactose malabsorption.
With caution
Risk of bleeding
The use of the drug is not recommended for liver diseases accompanied by disorders in the blood coagulation system and a clinically significant risk of bleeding. It is necessary to stop using the drug if severe bleeding occurs.
If a complication occurs in the form of bleeding, drug therapy should be stopped; it is also necessary to establish the source of bleeding. Possible options for stopping bleeding include surgical hemostasis or transfusion of fresh frozen plasma; for life-threatening conditions that cannot be controlled using the above methods, administration of prothrombin complex concentrate (PCC) or recombinant coagulation factor VIIa can be considered. In healthy volunteers, reversibility of the pharmacodynamic effects of Eliquis®, as determined by the thrombin generation test, was observed after infusion of a COC containing 4 coagulation factors. However, there is no experience in the clinical use of COC containing 4 coagulation factors to stop bleeding in patients receiving Eliquis®. There is currently no experience with the use of recombinant factor VIIa in patients receiving apixaban therapy.
Caution should be exercised when using apixaban simultaneously with nonsteroidal anti-inflammatory drugs (NSAIDs) (including acetylsalicylic acid) due to the fact that these drugs increase the risk of bleeding.
Concomitant use of Eliquis® and acetylsalicylic acid (ASA) in elderly patients requires caution due to a potentially higher risk of bleeding. In patients with atrial fibrillation and conditions requiring mono or dual antiplatelet therapy, a careful risk-benefit assessment should be performed before prescribing apixaban.
Side Effects
Side Effects
Common adverse reactions were bleeding, bruising, epistaxis, and hematoma (see adverse reaction profile and frequency by indication below). The frequency of adverse reactions is understood as: often – ≥ 1/100, <1/10, infrequently - ≥ 1/1000, <1/100. rarely - ≥ 1/10000, < 1/1000.
* There were no cases of generalized pruritus in the CV185057 study (long-term VTE prophylaxis).
The use of Eliquis® may be associated with an increased risk of bleeding (including hidden) from any organ or tissue, which in turn can lead to the development of posthemorrhagic anemia. Symptoms, signs, and severity will vary depending on the source of the bleeding and the extent or extent of the bleeding.
Interaction
Interaction
Inhibitors of the isoenzyme CYP3A4 and P-glycoprotein
A combination of apixaban with ketoconazole (at a dose of 400 mg, 1 time per day), which is a powerful inhibitor of both the CYP3A4 isoenzyme, Hak and P-glycoprotein. led to an increase in the average AUC value of apixaban by 2 times and the average Cmax value by 1.6 times.
The use of Eliquis is not recommended in patients receiving concomitant systemic treatment with potent inhibitors of the CYP3A4 isoenzyme and P-glycoprotein, such as azole antimycotics (for example, ketoconazole, itraconazole, voriconazole and posaconazole) and HIV protease inhibitors (for example, ritonavir) (see section “Special Instructions”).
Drugs other than potent inhibitors of CYP3A4 and P-glycoprotein (e.g. diltiazem, paproxen, clarithromycin, amiodarone, verapamil, quinidine) are likely to increase plasma concentrations of apixaban to a lesser extent. No dose adjustment of apixaban is required when combined with other drugs that are not potent inhibitors of the CYP3A4 isoenzyme and P-glycoprotein. For example, diltiazem (a moderate inhibitor of the CYP3A4 isoenzyme and a weak inhibitor of P-glycoprotein) at a dose of 360 mg 1 time per day led to an increase in the average AUC values of apixaban by 1.4 times and the average Cmax values by 1.3 times. Naproxen (an inhibitor of P-glycoprotein, but not the CYP3A4 isoenzyme) when used at a dose of 500 mg caused an increase in the average AUC and Cmax of apixaban by 1.5 and 1.6 times, respectively. Clarithromycin (a P-glycoprotein inhibitor and a potent inhibitor of the CYP3A4 isoenzyme) at a dose of 500 mg 2 times a day increased the average AUC and Cmax of apixaban by 1.6 and 1.3 times, respectively.
Inducers of the isoenzyme CYP3A4 and P-glycoprotein
The combination of apixaban with rifampicin (a potent inducer of CYP3A4 and P-glycoprotein) resulted in a decrease in the mean AUC and Cmax of apixaban by approximately 54% and 42%, respectively. Apparently, the combination of apixaban with other potent inducers of the CYP3A4 isoenzyme and P-glycoprotein (in particular, phenytoin, carbamazepine, phenobarbital or St. John’s wort preparations) may also lead to a decrease in the concentration of apixaban in the blood plasma (by approximately 50%). Dose adjustment of apixaban when combined with drugs in this group is not required when prescribed according to the following indications: prevention of thromboembolism after joint replacement, prevention of strokes and systemic thromboembolism in non-valvular atrial fibrillation and prevention of relapses of deep vein thrombosis, pulmonary embolism, however, these drugs should be combined with caution. When used for the treatment of deep vein thrombosis and pulmonary embolism, the combined use of apixaban and potent inducers of the CYP3A4 isoenzyme and P-glycoprotein is not recommended, as the effectiveness may be reduced (see section “Special Instructions”).
Anticoagulants, platelet aggregation inhibitors and NSAIDs
Due to the high risk of bleeding, simultaneous use with any other anticoagulants is contraindicated (see section “Contraindications”). After co-administration of enoxaparin (single dose, 40 mg) and apixaban (single dose, 5 mg), an additive effect of these drugs on FXa activity was noted.
There were no signs of pharmacokinetic or pharmacodynamic interaction of apixaban with acetylsalicylic acid (at a dose of 325 mg, 1 time per day) in healthy people.
Combining apixaban with clopidogrel (at a dose of 75 mg, once a day) or a combination of clopidogrel (75 mg) and acetylsalicylic acid (162 mg, once a day) or prasugrel (60 mg followed by a dose of 10 mg once a day) in a phase I clinical trial did not lead to an increase in bleeding time or a more pronounced inhibition of platelet aggregation compared with the use of these antiplatelet agents in monotherapy. The increase in blood coagulation parameters (prothrombin time, INR and APTT) corresponded to the effects of apixaban when used in monotherapy.
Naproxen (500 mg), which is a P-glycoprotein inhibitor, increases the mean AUC and Cmax of apixaban by 1.5 and 1.6 times, respectively. A corresponding increase in blood clotting parameters was observed with apixaban. There were no changes in the effect of naproxen on arachidonic acid-induced platelet aggregation, and no clinically significant increase in bleeding time was observed after coadministration of apixaban and naproxen. Despite these data, some patients may experience a greater pharmacodynamic response after coadministration of apixaban with other antiplatelet agents. Eliquis should be used with caution concomitantly with NSAIDs (including acetylsalicylic acid) as these drugs usually increase the risk of bleeding. There was a statistically significant increase in the risk of major bleeding according to the International Society on Thrombosis and Haemostasis (ISTH) criteria when using triple therapy with apixaban, acetylsalicylic acid (ASA) and clopidogrel in a clinical trial in patients with acute coronary syndrome at high risk of thrombosis and several concomitant cardiac and non-cardiac diseases.
It is not recommended to simultaneously use drugs that may be associated with the development of serious bleeding, such as thrombolytic drugs, glycoprotein IIb/IIIa receptor antagonists, thienopyridines (for example, clopidogrel), dipyridamole, dextran and sulfinpyrazone.
Combination with other drugs
There were no clinically significant pharmacokinetic or pharmacodynamic interactions of apixaban with atenolol or famotidine. Combining apixaban (at a dose of 10 mg) with atenolol (at a dose of 100 mg) did not lead to the development of clinically significant changes in the pharmacokinetic parameters of apixaban, but it was accompanied by a decrease in the average AUC and Cmax values of apixaban by 15% and 18%, respectively, compared with the monotherapy regimen. Administration of apixaban (at a dose of 10 mg) with famotidine (at a dose of 40 mg) had no effect on the AUC or Cmax values of apixaban.
Effect of apixaban on the pharmacokinetics of other drugs
In in vitro studies, apixaban did not inhibit the activity of the isoenzymes CYP1A2, CYP2A6, CYP2B6, and CYP2C8. CYP2C9, CYP2D6 or CYP3A4 (inhibitory concentration (IC50) > 45 µmol/l) and at the same time weakly suppressed the activity of the CYP2C19 isoenzyme (IC50 > 20 µmol/l) at a concentration significantly higher than the maximum concentration of the drug in the blood plasma during its clinical use. Apixaban is not an inducer of the isoenzymes CYP1A2, CYP2B6, CYP3A4/5 at concentrations up to 20 µmol/l. In this regard, it is expected that when used together it will not affect the clearance of drugs metabolized by these isoenzymes. In addition, apixaban does not significantly inhibit P-glycoprotein activity.
In studies in healthy volunteers, apixaban did not significantly alter the pharmacokinetics of digoxin, naproxen, or atenolol.
Digoxin
When taking apixaban (at a dose of 20 mg once a day) and digoxin (at a dose of 0.25 mg once a day), which is a P-gp substrate, the AUC or Cmax values of digoxin did not change. Thus, apixaban does not inhibit the transport of P-glycoprotein substrates.
Naproxen
Concomitant use of single doses of apixaban (10 mg) and naproxen (500 mg), a commonly used NSAID, did not affect the AUC and Cmax of naproxen.
Atenolol
Co-administration of a single dose of apixaban (10 mg) and atenolol (100 mg), a widely used beta-blocker, did not alter the pharmacokinetics of atenolol.
Activated carbon
Taking activated carbon reduces the effect of apixaban (see section “Overdose”).
Overdose
Overdose
No antidote is known. In case of overdose, the risk of bleeding increases. Hemodialysis is not expected to be an effective treatment for apixaban overdose.
In controlled clinical studies, apixaban was administered orally to healthy volunteers in doses up to 50 mg/day for 3 to 7 days (25 mg, twice daily, for 7 days or 50 mg, once daily, for 3 days); There were no clinically significant undesirable effects.
In case of overdose of this drug, the use of activated carbon may be considered.
When healthy volunteers were administered activated charcoal 2 and 6 hours after taking apixaban 20 mg, the area under the concentration-time curve (AUC) for apixaban decreased by 50% and 27%, respectively (Cmax did not change). The half-life of apixaban decreased from 13.4 to 5.3 and 4.9 hours, respectively.
Storage conditions
Storage conditions
Store at a temperature not exceeding 30 °C.
Keep out of the reach of children!
Shelf life
Shelf life
3 years. Do not use after expiration date.
Manufacturer
Manufacturer
Pfizer Ireland Pharmaceuticals, Ireland
Additional information
| Shelf life | 3 years. Do not use after the expiration date. |
|---|---|
| Conditions of storage | Store at temperatures not exceeding 30 ° C. Keep out of reach of children! |
| Manufacturer | Pfizer Ireland Pharmaceuticals, Ireland |
| Medication form | pills |
| Brand | Pfizer Ireland Pharmaceuticals |
Other forms…
Related products
Buy Eliquis, 2.5mg 20 pcs. with delivery to USA, UK, Europe and over 120 other countries.