No products in the cart.
Duloxetine Canon, 60 mg 28 pcs
€77.12 €64.27
Description
Pharmgroup:
antidepressant.
Pharmic action:
Inhibits serotonin and noadrenaline reuptake, resulting in increased serotonergic and noradrenergic neurotransmission in the CNS.
It weakly inhibits dopamine uptake without significant affinity for histaminergic, dopaminergic, cholinergic and adrenergic receptors.
Duloxetine has a central mechanism of pain syndrome suppression, which is primarily manifested by an increase in the threshold of pain sensitivity in pain syndrome of neuropathic etiology.
Indications
Indications
Depression, diabetic peripheral neuropathy (painful form).
Pharmacological effect
Pharmacological effect
Pharmaceutical group:
antidepressant.
Pharmaceutical action:
Inhibits the reuptake of serotonin and noadrenaline, resulting in increased serotonergic and noradrenergic neurotransmission in the central nervous system.
Weakly inhibits dopamine uptake, without having significant affinity for histaminergic, dopaminergic, cholinergic and adrenergic receptors.
Duloxetine has a central mechanism for suppressing pain, which is primarily manifested by an increase in the threshold of pain sensitivity in pain of neuropathic etiology.
Special instructions
Special instructions
A systematic evaluation of the drug at doses above 120 mg has not been carried out.
Cases of mydriasis have been observed when taking duloxetine, so caution should be exercised when prescribing duloxetine to patients with intraocular hypertension or in individuals at risk of developing acute angle-closure glaucoma.
In patients with severe chronic renal failure (creatinine clearance less than 30 ml/min) or severe liver failure, an increase in plasma duloxetine concentrations is observed. If duloxetine is clinically justified in such patients, lower initial dosages should be used.
With depression, there is a possibility of suicide attempts, which may persist until stable remission occurs. Careful monitoring of patients at risk is necessary.
The drug should be discontinued gradually to avoid withdrawal syndrome.
Due to insufficient experience with the use of duloxetine during pregnancy, the drug should be prescribed during pregnancy only if the potential benefit to the mother significantly outweighs the potential risk to the fetus. Patients should be warned that if pregnancy occurs or is planned during treatment with duloxetine, they should inform their doctor.
Due to the lack of experience with the use of duloxetine in women during lactation, breastfeeding is not recommended during duloxetine therapy.
During studies of duloxetine, no impairment of psychomotor reactions, cognitive functions and memory was identified. However, taking the drug may be accompanied by drowsiness. In this regard, patients taking duloxetine should exercise caution when engaging in potentially hazardous activities that require increased concentration and speed of psychomotor reactions, incl. driving a car.
Active ingredient
Active ingredient
Duloxetine
Composition
Composition
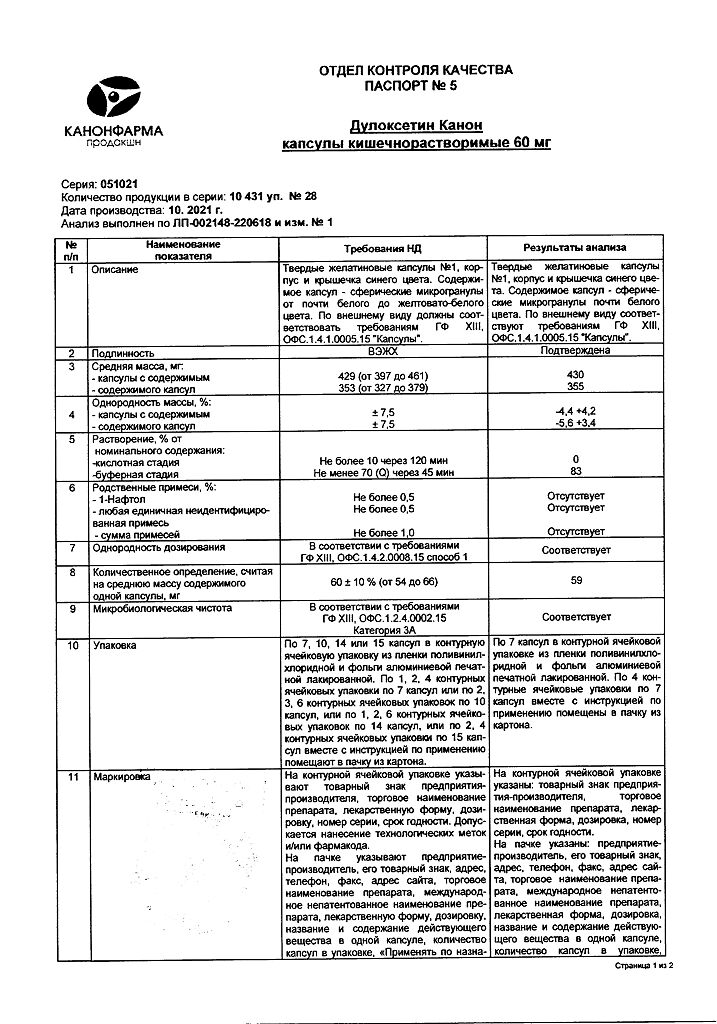
Enteric-soluble hard gelatin capsules No. 1, blue body and cap; the contents of the capsules are spherical microgranules from almost white to yellowish-white.
1 caps. duloxetine (hydrochloride form) 60 mg
Excipients:
hypromellose E5 21.08 mg,
hypromellose HP55 31.02 mg,
starch 88.18 mg,
mannitol 94.6 mg,
sodium lauryl sulfate 10.44 mg,
sucrose 34.92 mg,
titanium dioxide 2.3 mg,
cetyl alcohol 3.1 mg.
Composition of the capsule shell:
gelatin,
titanium dioxide,
patented blue dye V.
Contraindications
Contraindications
Hypersensitivity, liver diseases accompanied by liver failure, simultaneous use of non-selective irreversible MAO inhibitors, potent CYP1A2 inhibitors (fluvoxamine, ciprofloxacin, enoxacin), severe chronic renal failure (creatinine clearance less than 30 ml/min), uncontrolled arterial hypertension, lactation period, age under 18 years (no experience with use).
For dosage forms containing sucrose (additionally): congenital fructose intolerance, glucose-galactose malabsorption, sucrose-isomaltase deficiency.
With caution. Mania and bipolar disorder (including a history), seizures (including a history), intraocular hypertension or the risk of developing an acute attack of angle-closure glaucoma, a history of suicidal thoughts and attempts, an increased risk of hyponatremia (elderly patients, cirrhosis of the liver, dehydration, taking diuretics), pregnancy.
Side Effects
Side Effects
Frequency: very common (1/10 m), common (more than 1/100 and less than 1/10), uncommon (1/1000 and less than 1/100), rare (1/10,000 and less than 1/1000), very rare (less than 1/10,000), frequency unknown (cannot be estimated from available data).
From the nervous system: very often – headache, drowsiness; often – dizziness, tremor, lethargy, paresthesia, insomnia, unusual dreams, anxiety, agitation; uncommon – dyskinesia, poor sleep quality, nervousness, myoclonus, impaired concentration, sleep disturbance, apathy, disorientation, bruxism; rarely – mania, aggressiveness, anger; frequency unknown – serotonin syndrome, convulsions, akathisia, psychomotor restlessness, extrapyramidal syndrome, suicide attempts, suicidal thoughts, hallucinations.
From the digestive system: very often – nausea, dryness of the oral mucosa; often – diarrhea, constipation, vomiting, dyspepsia, flatulence, abdominal pain; uncommon – gastroenteritis, stomatitis, gastritis, belching, taste disturbance, hepatitis, acute liver failure, increased activity of liver transaminases (ALT, AST, alkaline phosphatase); rarely – unpleasant odor when breathing, unchanged blood in the stool; frequency unknown – gastrointestinal bleeding, jaundice, liver failure.
From the genitourinary system: often – erectile dysfunction, decreased libido, changes in the ability to experience the feeling of orgasm; uncommon – urinary retention, intermittent urination, dysuria, nocturia, polyuria, decreased urine flow, impaired sexual function, impaired ejaculation, incl. delayed ejaculation, gynecological bleeding; rarely – symptoms of menopause; frequency unknown – change in urine odor.
From the cardiovascular system: often – palpitations, “flushes” of blood; infrequently – tachycardia, fainting and orthostatic hypotension (which were reported only at the beginning of treatment), increased blood pressure, cold extremities; rarely – supraventricular arrhythmia, predominantly atrial fibrillation; frequency unknown – hypertensive crisis.
From the senses: often – blurred visual perception, tinnitus; uncommon – blurred vision, mydriasis, vertigo, ear pain; rarely – glaucoma.
From the respiratory system: often – yawning; infrequently – nosebleeds, a feeling of constriction in the throat.
From the skin: often – rash, increased sweating, night sweats, infrequently – photosensitivity, increased tendency to subcutaneous hemorrhages, contact dermatitis, urticaria, cold sweat; frequency unknown – angioedema, Stevens-Johnson syndrome.
From the musculoskeletal system: often – muscle spasm, musculoskeletal pain, muscle stiffness; infrequently – muscle twitching; rarely – trismus.
From the endocrine system: rarely – hypothyroidism.
Metabolism: often – loss of appetite; uncommon – hyperglycemia (reported mainly in patients with diabetes mellitus); rarely – dehydration, syndrome of inappropriate secretion of antidiuretic hormone, hyponatremia.
Infections: uncommon – laryngitis.
Other: often – fatigue, weight loss; infrequently – weight gain, malaise, gait disturbance, sensory disturbance, feeling of cold, feeling of warmth, thirst, chills, increased CPK; rarely – hypercholesterolemia; frequency unknown – chest pain.
Allergic reactions: uncommon – hypersensitivity reactions; rarely – anaphylactoid reactions.
In case of abrupt withdrawal – withdrawal syndrome, the most common symptoms of which were dizziness, sensory disturbances (including paresthesia), sleep disturbances (including insomnia, intense dreams), agitation or anxiety, nausea and/or vomiting, tremor, headache, irritability, diarrhea, hyperhidrosis, vertigo.
Interaction
Interaction
Concomitant use of duloxetine (60 mg 2 times daily) did not have a significant effect on the pharmacokinetics of theophylline, which is metabolized by CYP1A2.
Concomitant use of duloxetine with potential CYP1A2 inhibitors may result in increased duloxetine concentrations. The CYP1A2 inhibitor fluvoxamine (100 mg once daily) reduces the plasma clearance of duloxetine by approximately 77% and increases the AUC by 6-fold, so duloxetine should not be used in combination with potential CYP1A2 inhibitors such as fluvoxamine.
Duloxetine is a moderate inhibitor of CYP2D6. When taking duloxetine at a dose of 60 mg 2 times a day with a single dose of desipramine (CYP2D6 substrate), the AUC of desipramine increases 3-fold. Co-administration of duloxetine (40 mg 2 times a day) increases the AUC of tolterodine (2 mg 2 times a day) by 71%, but does not affect the pharmacokinetics of the 5-hydroxyl metabolite. Caution should be exercised when using duloxetine with drugs that are metabolized mainly by the CYP2D6 system and have a narrow therapeutic index.
Concomitant use of duloxetine with potential CYP2D6 inhibitors may result in increased duloxetine concentrations.
Paroxetine (20 mg once daily) reduces the clearance of duloxetine by approximately 37%. Caution should be used when using duloxetine with CYP2D6 inhibitors.
Caution should be exercised when using duloxetine simultaneously with other drugs that affect the central nervous system (including benzodiazepines, antipsychotic drugs, phenobarbital, antihistamines with sedative effects, ethanol), especially with a similar mechanism of action.
The simultaneous use of duloxetine with drugs that are highly bound to plasma proteins can lead to an increase in the concentration of free fractions of both drugs.
In patients receiving a serotonin reuptake inhibitor in combination with non-selective irreversible MAO inhibitors, there have been cases of severe adverse reactions (hyperthermia, muscle rigidity, myoclonus, peripheral disturbances with possible sharp fluctuations in vital signs and changes in mental status, including severe agitation leading to delirium and coma), sometimes with death. These reactions have also been observed in patients who were discontinued with a serotonin reuptake inhibitor shortly before being prescribed an MAO inhibitor. In some cases, patients experienced symptoms consistent with neuroleptic malignant syndrome. The effects of combined use of duloxetine and MAO inhibitors have not been evaluated in either humans or animals. Therefore, given the fact that duloxetine is an inhibitor of both serotonin and norepinephrine, it is not recommended to take duloxetine in combination with non-selective irreversible MAO inhibitors or for at least 14 days after their discontinuation. Based on the T1/2 duration of duloxetine, a break of at least 5 days should be taken after stopping duloxetine before taking MAO inhibitors. With respect to selective reversible MAO inhibitors, such as moclobemide, the risk of developing serotonin syndrome is low, however, simultaneous use of the drug with selective reversible MAO inhibitors is not recommended.
Anticoagulants and antiplatelet drugs – the risk of bleeding. Cases of increased INR have been reported when used concomitantly with warfarin.
In rare cases, the development of serotonin syndrome has been reported when using SSRIs (including paroxetine, fluoxetine) with serotonergic drugs. Caution should be exercised when duloxetine is used concomitantly with serotonergic antidepressants such as SSRIs, tricyclic antidepressants such as clomipramine and amitriptyline, St. John’s wort, venlafaxine or triptans, tramadol, pethidine and tryptophan.
Overdose
Overdose
Symptoms: vomiting and loss of appetite, tremor, clonic convulsions, ataxia. Treatment: symptomatic and supportive. Monitoring of cardiovascular system and other vital signs. A specific antidote is not known.
Several cases of overdose have been reported with simultaneous oral administration of up to 1400 mg of the drug, which did not have fatal consequences.
Manufacturer
Manufacturer
Kanonpharma production CJSC, Russia
Additional information
| Manufacturer | Kanonfarma Production ZAO, Russia |
|---|---|
| Medication form | enteric capsules |
| Brand | Kanonfarma Production ZAO |
Other forms…
Related products
Buy Duloxetine Canon, 60 mg 28 pcs with delivery to USA, UK, Europe and over 120 other countries.