No products in the cart.
Diuver, tablets 5 mg 20 pcs
€1.00
Out of stock
(E-mail when Stock is available)
Description
Pharmacotherapeutic group: diuretic agent.
AtCode C03CA04
Pharmacological properties
Pharmacodynamics
Indications
Indications
Active ingredient
Active ingredient
Composition
Composition
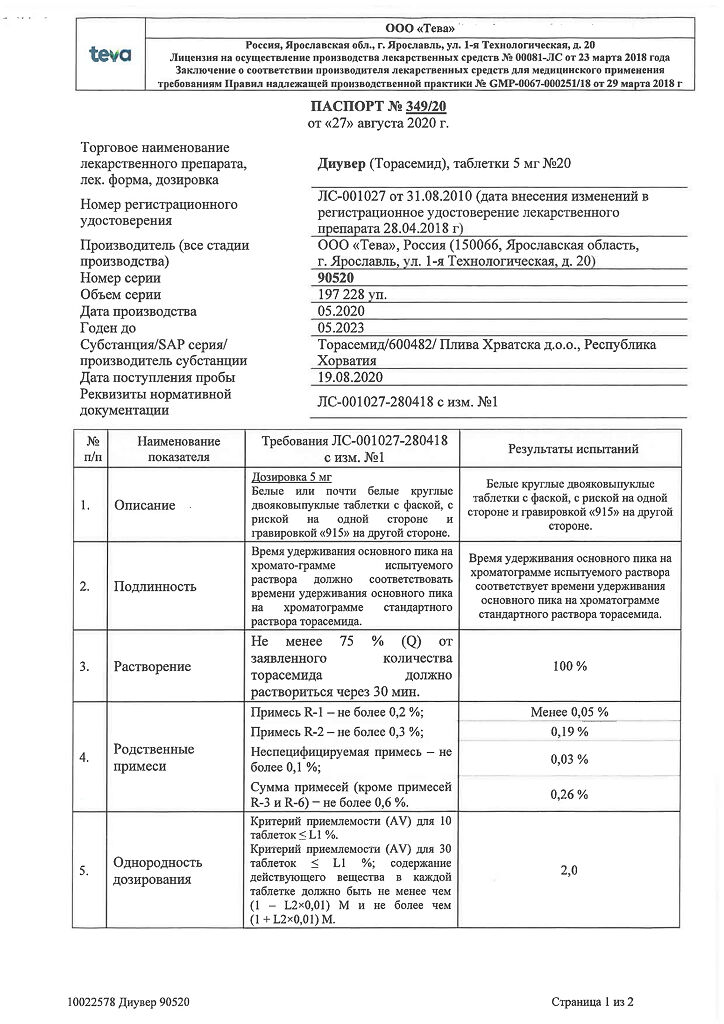
1 tablet 5 mg contains:
active ingredient: torasemide 5.00 mg;
excipients: lactose monohydrate 58.44 mg, corn starch 14.56 mg, sodium carboxymethyl starch 0.80 mg, colloidal anhydrous silica 0.60 mg, magnesium stearate 0.60 mg.
1 tablet 10 mg contains:
acting substance: torasemide 10.00 mg;
complementary substances: lactose monohydrate 116.88 mg, corn starch 29.12 mg, sodium carboxymethyl starch 1.60 mg, colloidal anhydrous silica 1.20 mg, magnesium stearate 1.20 mg.
How to take, the dosage
How to take, the dosage
Overly, once a day, after breakfast, with a small amount of water.
Oedematous syndrome of different genesis, including. In chronic heart failure, liver, lung and kidney disease
The usual therapeutic dose is 5 mg orally once daily. If necessary, the dose should be gradually increased to 20-40 mg once daily, in some cases up to 200 mg per day. The drug is prescribed for a long period or until the edema disappears.
Arterial hypertension
The initial dose is 2.5 mg (1/2 tablet of 5 mg) once daily. If necessary, the dose may be increased to 5 mg once daily.
Elderly patients do not need dose adjustment.
Interaction
Interaction
Special Instructions
Special Instructions
Synopsis
Synopsis
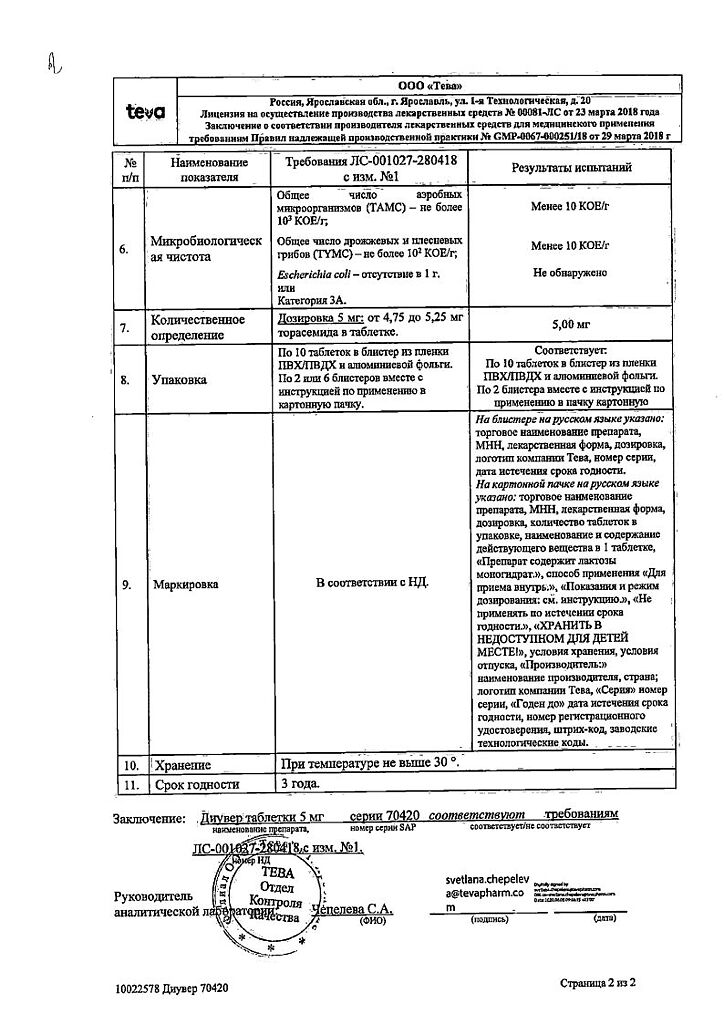
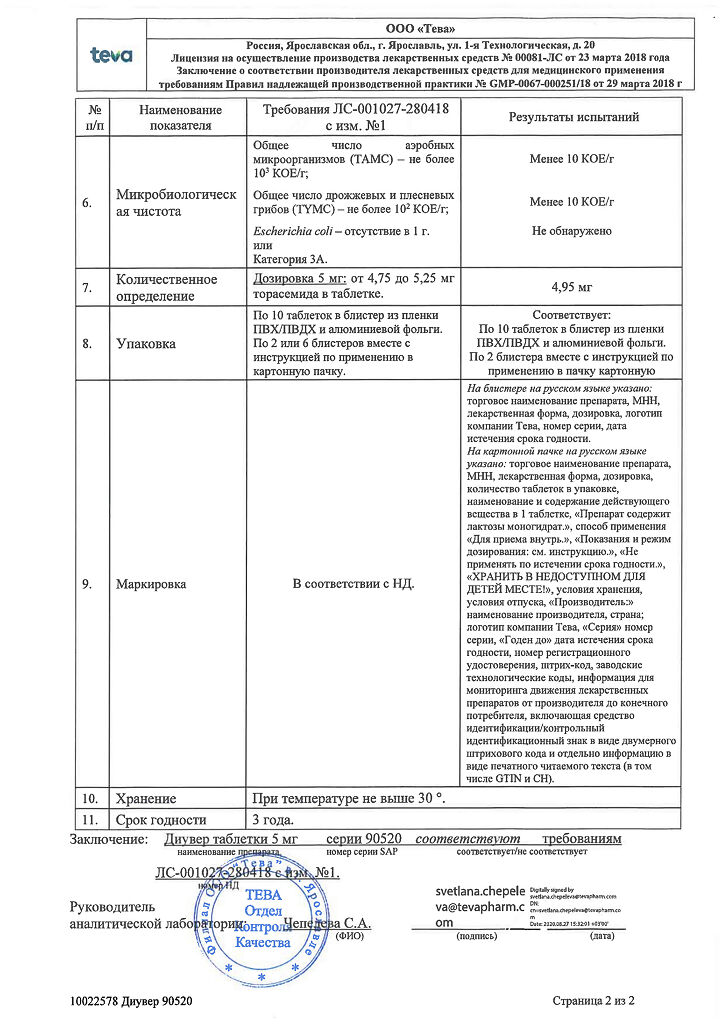
Dosage 5 mg:
White or almost white round biconvex tablets with a bevel on one side and engraved “915” on the other side.
Dosage 10 mg:
White or almost white round biconvex tablets with a bevel, with a groove on one side and engraving “916” on the other side.
Contraindications
Contraindications
Side effects
Side effects
Water-electrolyte and acid-base balance: hyponatremia, hypochloremia, hypokalemia, hypomagnesemia, hypocalcemia, metabolic alkalosis. Symptoms indicative of the development of electrolyte and acid-base state disorders may include headache, confusion, seizures, tetany, muscle weakness, heart rhythm disorders and dyspeptic disorders; hypovolemia and dehydration (more common in elderly patients), which may lead to hemoconcentration with a tendency to develop thrombosis.
Cardiovascular system disorders: excessive decrease in blood pressure, orthostatic hypotension, collapse, tachycardia, arrhythmias, decreased circulating blood volume.
Metabolic side: Hypercholesterolemia, hypertriglyceridemia; transient increase in blood creatinine and urea concentrations; increase in blood uric acid concentration, which may cause or worsen manifestations of gout; decrease in glucose tolerance (possible manifestation of latent diabetes).
Urinary system disorders: oliguria, acute urinary retention (e.g., in prostatic hyperplasia, narrowing of the urethra, hydronephrosis); interstitial nephritis, hematuria, decreased potency.
Gastrointestinal tract disorders: nausea, vomiting, diarrhea, intrahepatic cholestasis, increased activity of “liver” enzymes, acute pancreatitis.
Central nervous system, hearing: disorder of hearing, usually reversible, and/or tinnitus, especially in patients with renal failure or hypoproteinemia (nephrotic syndrome), paresthesias.
Skin side: cutaneous itching, urticaria, other rashes or bullous skin lesions, erythema polymorphicum, exfoliative dermatitis, purpura, fever, vasculitis, eosinophilia, photosensitization; severe anaphylactic or anaphylactoid reactions up to shock, which so far have only been described after intravenous administration.
Peripheral blood: thrombocytopenia; leukopenia; agranulocytosis, aplastic or hemolytic anemia.
Overdose
Overdose
Pregnancy use
Pregnancy use
Toracemide has no teratogenic effect and fetotoxicity, it penetrates the placental barrier, causing water-electrolyte metabolism disorders and thrombocytopenia in the fetus.
Diuver during pregnancy should be used only if the benefit to the mother outweighs the possible potential risk to the fetus, only under medical supervision and only in the lowest possible doses.
It is unknown whether thoracemide penetrates into the breast milk. If it is necessary to use the drug Diuver during lactation, breastfeeding should be stopped.
Similarities
Similarities
Additional information
| Shelf life | 3 years. Do not use after the expiration date. |
|---|---|
| Conditions of storage | At a temperature not exceeding 25°C. Keep out of reach of children! |
| Manufacturer | Teva LLC, Russia |
| Medication form | pills |
| Brand | Teva LLC |
Related products
Buy Diuver, tablets 5 mg 20 pcs with delivery to USA, UK, Europe and over 120 other countries.