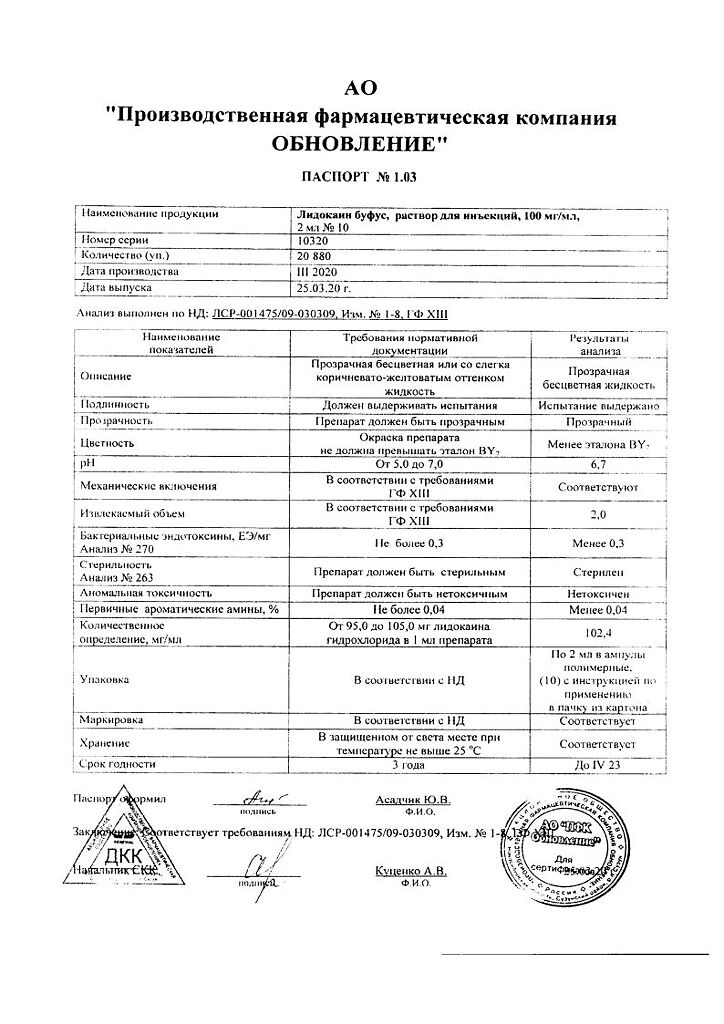
No products in the cart.
Dexamethasone, tablets 0.5 mg 56 pcs
€1.96 €1.78
Description
Dexamethasone is a synthetic glucocorticosteroid (GCS), methylated fluoroprednisolone derivative. It has anti-inflammatory, anti-allergic, desensitizing, immunosuppressive, antishock and antitoxic effects.
Inhibits secretion of thyroid hormone and follicle stimulating hormone.
Increases central nervous system excitability, decreases the number of lymphocytes and eosinophils, increases the number of erythrocytes (stimulates erythropoietin production).
Interacts with specific cytoplasmic receptors and forms a complex penetrating into cell nucleus, stimulates synthesis of matrix ribonucleic acid (mRNA); the latter induces formation of proteins, including lipocortin, mediating cellular effects. Lipocortin inhibits phospholipase A2, inhibits the release of arachidonic acid and inhibits the synthesis of endoperoxins, prostaglandins, leukotrienes, contributing to the processes of inflammation, allergy and others.
Effect on protein metabolism: reduces the amount of protein in the plasma (at the expense of globulins) with an increase in albumin/globulin ratio, increases the synthesis of albumin in the liver and kidneys; increases protein catabolism in muscle tissue.
Effect on lipid metabolism: increases the synthesis of higher fatty acids and triglycerides, redistributes fat (fat accumulation mainly in the shoulder girdle, face, abdomen), leads to hypercholesterolemia.
Effect on carbohydrate metabolism: increases absorption of carbohydrates from the gastrointestinal tract (GIT), increases the activity of glucose-6-phosphatase, leading to increased glucose flow from the liver into the blood, increases the activity of phosphoenolpyruvate carboxylase and aminotransferase synthesis, which leads to activation of gluconeogenesis.
Effect on water-electrolyte metabolism: it retains sodium ions and water in the body, stimulates excretion of potassium ions (mineralocorticosteroid activity), reduces the absorption of calcium ions from the GIT, “washes” calcium ions from the bones, increases excretion of calcium ions by kidneys.
Anti-inflammatory activity is associated with inhibition of eosinophils release of inflammatory mediators, induction of lipocortin formation and reduction of hyaluronic acid-producing mast cells number, capillary permeability reduction, stabilization of cell and organelle membranes (especially lysosomal).
. An antiallergic effect is produced due to suppression of allergy mediators synthesis and secretion, inhibition of histamine and other bioactive substances release from sensitized mast cells and basophils, reduction of circulating basophils number, suppression of lymphoid and connective tissue development, reduction of T- and B-lymphocytes quantity, mast cells, reduction of effector cells sensitivity to allergy mediators, suppression of antibody formation, change in the organism immune response.
In chronic obstructive pulmonary disease action is mainly based on inhibition of inflammatory processes, inhibition or prevention of mucous membrane edema, inhibition of eosinophilic infiltration of submucous layer of bronchial epithelium, deposition of circulating immune complexes in bronchial mucosa, and inhibition of mucous membrane erosion and desquamation. It increases sensitivity of small and medium caliber bronchial beta-adrenoreceptors to endogenous catecholamines and exogenous sympathomimetics, decreases bronchial secretion viscosity by inhibiting or reducing its production.
Antishock and antitoxic action is associated with increase of arterial pressure (due to increase in concentration of circulating catecholamines and restoration of adrenoreceptors sensitivity to them as well as vasoconstriction), reduction of vascular wall permeability, membrane-protective properties, activation of liver enzymes involved in metabolism of endo- and xenobiotics.
Immunosuppressive effect is caused by inhibition of cytokine release (interleukin-1, interleukin-2, interferon gamma) from lymphocytes and macrophages.
Inhibits the synthesis and secretion of adrenocorticotropic hormone (ACTH), and secondary the synthesis of endogenous GCS.
Peculiarities of action are significant inhibition of pituitary function and almost complete absence of mineralocorticosteroid activity. Doses of 1-1.5 mg/day inhibit adrenal cortex; biological half-life is 32-72 hours (duration of inhibition of hypothalamic-pituitary-adrenal cortical layer system).
In terms of glucocorticoid activity 0.5 mg of dexamethasone corresponds to approximately 3.5 mg of prednisolone, 15 mg of hydrocortisone or 17.5 mg of cortisone for oral dosage forms.
Pharmacokinetics
Absorption
After oral administration is quickly and completely absorbed, the maximum concentration of dexamethasone in blood plasma is 1-2 hours.
Distribution
In the blood it is bound (60 – 70%) with the specific carrier protein – transcortin. Easily passes through histohematic barriers (including the blood-brain barrier and the placental barrier).
Metabolism
Metabolized in liver (mainly by conjugation with glucuronic and sulfuric acids) to inactive metabolites.
Excretion
Excreted by the kidneys (a small amount of dexamethasone penetrates into breast milk). The elimination half-life is 3-5 hours.
Indications
Indications
Systemic connective tissue diseases (systemic lupus erythematosus, scleroderma, periarteritis nodosa, dermatomyositis, rheumatoid arthritis).
Acute and chronic inflammatory diseases of the joints: gouty and psoriatic arthritis, osteoarthritis (including post-traumatic), polyarthritis, glenohumeral periarthritis, ankylosing spondylitis (Bechterew’s disease), juvenile arthritis, Still’s syndrome in adults, bursitis, nonspecific tenosynovitis, synovitis and epicondylitis.
Rheumatic fever, acute rheumatic carditis.
Acute and chronic allergic diseases: allergic reactions to drugs and foods, serum sickness, urticaria, allergic rhinitis, angioedema, drug exanthema, hay fever.
Skin diseases: pemphigus, psoriasis, eczema, atopic dermatitis, diffuse neurodermatitis, contact dermatitis (affecting a large surface of the skin), toxicerma, seborrheic dermatitis, exfoliative dermatitis, toxic epidermal necrolysis (Lyell’s syndrome), bullous dermatitis herpetiformis, malignant exudative erythema (syndrome) Stevens-Johnson).
Brain edema (only after confirmation of symptoms of increased intracranial pressure by magnetic resonance or computed tomography) caused by a brain tumor and/or associated with surgery or radiation injury.
Allergic eye diseases: allergic corneal ulcers, allergic forms of conjunctivitis.
Inflammatory eye diseases: sympathetic ophthalmia, severe sluggish anterior and posterior uveitis, optic neuritis.
Primary or secondary adrenal insufficiency (including the condition after removal of the adrenal glands).
Congenital adrenal hyperplasia.
Kidney diseases of autoimmune origin (including acute glomerulonephritis); nephrotic syndrome.
Subacute thyroiditis.
Diseases of the hematopoietic organs – agranulocytosis, panmyelopathy, autoimmune hemolytic anemia, acute lymphoid and myeloid leukemia, lymphogranulomatosis, thrombocytopenic purpura, secondary thrombocytopenia in adults, erythroblastopenia (erythrocytic anemia), congenital (erythroid) hypoplastic anemia.
Lung diseases: acute alveolitis, pulmonary fibrosis, stage II-III sarcoidosis. Bronchial asthma (for bronchial asthma, the drug is prescribed only in severe cases, ineffectiveness or inability to take inhaled corticosteroids).
Tuberculous meningitis, pulmonary tuberculosis, aspiration pneumonia (in combination with specific chemotherapy).
Berylliosis, Loeffler’s syndrome (not amenable to other therapy).
Lung cancer (in combination with cytostatics).
Multiple sclerosis.
Gastrointestinal diseases: ulcerative colitis, Crohn’s disease, local enteritis.
Hepatitis.
Prevention of transplant rejection as part of complex therapy.
Hypercalcemia due to cancer, nausea and vomiting during cytostatic therapy.
Multiple myeloma.
Conducting a test for the differential diagnosis of hyperplasia (hyperfunction) and tumors of the adrenal cortex.
Pharmacological effect
Pharmacological effect
Dexamethasone is a synthetic glucocorticosteroid (GCS), a methylated derivative of fluoroprednisolone. It has anti-inflammatory, antiallergic, desensitizing, immunosuppressive, antishock and antitoxic effects.
Inhibits the secretion of thyroid-stimulating hormone and follicle-stimulating hormone.
Increases the excitability of the central nervous system, reduces the number of lymphocytes and eosinophils, increases the number of red blood cells (stimulates the production of erythropoietin).
Interacts with specific cytoplasmic receptors and forms a complex that penetrates the cell nucleus, stimulates the synthesis of matrix ribonucleic acid (mRNA); the latter induces the formation of proteins, including lipocortin, that mediate cellular effects. Lipocortin inhibits phospholipase A2, suppresses the release of arachidonic acid and suppresses the synthesis of endoperoxides, prostaglandins, leukotrienes, which contribute to inflammation, allergies and others.
Effect on protein metabolism: reduces the amount of protein in plasma (due to globulins) with an increase in the albumin/globulin ratio, increases albumin synthesis in the liver and kidneys; enhances protein catabolism in muscle tissue.
Effect on lipid metabolism: increases the synthesis of higher fatty acids and triglycerides, redistributes fat (accumulation of fat mainly in the shoulder girdle, face, abdomen), leads to the development of hypercholesterolemia.
Effect on carbohydrate metabolism: increases the absorption of carbohydrates from the gastrointestinal tract (GIT); increases the activity of glucose-6-phosphatase, leading to an increase in the flow of glucose from the liver into the blood; increases the activity of phosphoenolpyruvate carboxylase and the synthesis of aminotransferases, which leads to the activation of gluconeogenesis.
Effect on water-electrolyte metabolism: retains sodium ions and water in the body, stimulates the excretion of potassium ions (mineralocorticosteroid activity), reduces the absorption of calcium ions from the gastrointestinal tract, “washes out” calcium ions from the bones, increases the excretion of calcium ions by the kidneys.
The anti-inflammatory effect is associated with inhibition of the release of inflammatory mediators by eosinophils; inducing the formation of lipocortins and reducing the number of mast cells that produce hyaluronic acid; with a decrease in capillary permeability; stabilization of cell membranes and organelle membranes (especially lysosomal ones).
The antiallergic effect develops as a result of suppression of the synthesis and secretion of allergy mediators, inhibition of the release of histamine and other biologically active substances from sensitized mast cells and basophils, a decrease in the number of circulating basophils, suppression of the development of lymphoid and connective tissue, a decrease in the number of T and B lymphocytes, mast cells, a decrease in the sensitivity of effector cells to allergy mediators, inhibition of antibody formation, changes in the immune response body.
In chronic obstructive pulmonary disease, the effect is based mainly on inhibition of inflammatory processes, inhibition of development or prevention of swelling of the mucous membranes, inhibition of eosinophilic infiltration of the submucosal layer of the bronchial epithelium, deposition of circulating immune complexes in the bronchial mucosa, as well as inhibition of erosion and desquamation of the mucous membrane. Increases the sensitivity of beta-adrenergic receptors of small and medium caliber bronchi to endogenous catecholamines and exogenous sympathomimetics, reduces the viscosity of bronchial secretions due to inhibition or reduction of its production.
Antishock and antitoxic effects are associated with an increase in blood pressure (due to an increase in the concentration of circulating catecholamines and restoration of the sensitivity of adrenergic receptors to them, as well as vasoconstriction), a decrease in the permeability of the vascular wall, membrane protective properties, and activation of liver enzymes involved in the metabolism of endo- and xenobiotics.
The immunosuppressive effect is due to inhibition of the release of cytokines (interleukin-1, interleukin-2; interferon gamma) from lymphocytes and macrophages.
Suppresses the synthesis and secretion of adrenocorticotropic hormone (ACTH), and secondarily the synthesis of endogenous GCS.
The peculiarity of the action is significant inhibition of pituitary function and the almost complete absence of mineralocorticosteroid activity. Doses of 1-1.5 mg/day inhibit the adrenal cortex; biological half-life – 32-72 hours (duration of inhibition of the hypothalamus-pituitary-adrenal cortex system).
In terms of the strength of glucocorticoid activity, 0.5 mg of dexamethasone corresponds to approximately 3.5 mg of prednisolone, 15 mg of hydrocortisone or 17.5 mg of cortisone for oral dosage forms.
Pharmacokinetics
Absorption
After oral administration, it is quickly and completely absorbed, the maximum concentration of dexamethasone in the blood plasma is 1-2 hours.
Distribution
In the blood it binds (60–70%) to a specific carrier protein – transcortin. Easily passes through histohematic barriers (including the blood-brain and placental barriers).
Metabolism
Metabolized in the liver (mainly by conjugation with glucuronic and sulfuric acids) to inactive metabolites.
Removal
Excreted by the kidneys (a small amount of dexamethasone passes into breast milk). Half-life is 3-5 hours.
Special instructions
Special instructions
When prescribing dexamethasone for intercurrent infections, septic conditions and tuberculosis, it is necessary to simultaneously treat with specific antibacterial therapy when using the drug in patients with latent tuberculosis, lymphadenitis after BCG vaccination, polio, acute and chronic bacterial and parasitic infections; specific therapy in patients with gastric and/or intestinal ulcers, osteoporosis.
With daily use, atrophy of the adrenal cortex develops by 5 months of treatment.
May mask some symptoms of infections; It is useless to immunize during treatment.
With sudden withdrawal of GCS, especially in the case of previous use of high doses, GCS “withdrawal” syndrome occurs (not caused by hypocortisolism): loss of appetite, nausea, lethargy, generalized musculoskeletal pain, asthenia, and acute adrenal insufficiency may also occur (low blood pressure, arrhythmia, sweating, weakness, oligoanuria, vomiting, abdominal pain, diarrhea, hallucinations, fainting, coma).
After discontinuation, relative insufficiency of the adrenal cortex persists for several months. If stressful situations arise during this period, GCS is prescribed (according to indications), if necessary in combination with mineralocorticosteroids.
The dose of dexamethasone must be temporarily increased in stressful situations during therapy (surgery, trauma). A temporary increase in the dose of the drug in stressful situations is necessary both before and after stress.
In children, during long-term treatment, careful monitoring of the dynamics of growth and development is necessary. Children who during the treatment period were in contact with patients with measles or chickenpox are prescribed specific immunoglobulins prophylactically.
During treatment with dexamethasone (especially long-term), observation by an ophthalmologist, monitoring of blood pressure and water-electrolyte balance, as well as peripheral blood patterns and blood glucose concentrations are necessary. In order to reduce side effects, you can prescribe anabolic steroids, antacids, and also increase the intake of potassium ions in the body (eating foods rich in potassium and calcium, or taking potassium, calcium, and vitamin D supplements). Food should be rich in proteins, vitamins, and contain a small amount of fat, carbohydrates and salt.
In children during the growth period, GCS should be used only according to absolute indications and under especially careful supervision of the attending physician.
When using dexamethasone, there is a risk of developing severe anaphylactic reactions and bradycardia.
During drug therapy, the risk of activation of strongyloidiasis increases.
During drug therapy, careful monitoring of the condition of patients with CHF, uncontrolled arterial hypertension, injuries and ulcerative lesions of the cornea, and glaucoma is necessary.
Myasthenia gravis may worsen.
With the use of corticosteroids, changes in sperm motility are possible.
Taking the drug may mask the symptoms of “peritoneal irritation” in patients with perforation of the stomach or intestinal wall.
The effect of the drug is enhanced in patients with liver cirrhosis. It must be taken into account that in patients with hypothyroidism the clearance of dexamethasone is reduced, and in patients with thyrotoxicosis it is increased.
In patients with diabetes mellitus, blood glucose concentrations should be monitored and doses of hypoglycemic drugs adjusted if necessary.
Impact on the ability to drive vehicles and machinery
Considering the possible side effects during therapy with Dexamethasone, care must be taken when driving vehicles, operating machinery, or engaging in other activities that require increased concentration and speed of psychomotor reactions.
Active ingredient
Active ingredient
Dexamethasone
Composition
Composition
Active ingredient:
Dexamethasone – 0.0005 g,
Excipients: to obtain a tablet weighing 0.15 g
potato starch – 0.0340 g,
sucrose (sugar) – 0.1140 g,
stearic acid – 0.0015 g.
Pregnancy
Pregnancy
Dexamethasone crosses the placenta (can reach high concentrations in the fetus) and into breast milk.
During pregnancy, especially in the first trimester, or in women planning pregnancy, the use of Dexamethasone is indicated only if the expected therapeutic effect outweighs the risk of negative effects on the mother or fetus. GCS should be prescribed during pregnancy only for absolute indications.
With long-term therapy during pregnancy, the possibility of fetal damage cannot be excluded. If used in the third trimester of pregnancy, there is a risk of atrophy of the adrenal cortex in the fetus, which may require replacement therapy in the newborn.
If it is necessary to carry out treatment with the drug during breastfeeding, breastfeeding should be stopped.
Contraindications
Contraindications
For short-term use for “life-saving” indications, the only contraindication is hypersensitivity; systemic mycosis; simultaneous use of live and attenuated vaccines with immunosuppressive doses of the drug;
sucrose intolerance, isomaltase/sucrase deficiency, glucose-galactose malabsorption; breastfeeding period; children up to 3 years of age.
With caution
Parasitic and infectious diseases of a viral, fungal or bacterial nature (currently or recently suffered, including recent contact with a patient) – herpes simplex, herpes zoster (viremic phase), chicken pox, measles; amebiasis, strongyloidiasis (established or suspected); active and latent tuberculosis. Use for severe infectious diseases is permissible only against the background of specific antimicrobial therapy.
Pre- and post-vaccination period (8 weeks before and 2 weeks after vaccination), lymphadenitis after BCG vaccination. Immunodeficiency conditions (including acquired immunodeficiency syndrome or human immunodeficiency virus (HIV)).
Gastrointestinal diseases: peptic ulcer of the stomach and duodenum, esophagitis, gastritis, acute or latent peptic ulcer, recently created intestinal anastomosis, ulcerative colitis with the threat of perforation or abscess formation, diverticulitis.
Diseases of the cardiovascular system, including recent myocardial infarction (in patients with acute and subacute myocardial infarction, the necrosis focus may spread, the formation of scar tissue may slow down and, as a result, the heart muscle will rupture), decompensated chronic heart failure (CHF), arterial hypertension, hyperlipidemia.
Endocrine diseases – diabetes mellitus (including impaired carbohydrate tolerance), thyrotoxicosis, hypothyroidism, Itsenko-Cushing’s disease, obesity (stage III-IV).
Severe chronic renal and/or liver failure, nephrourolithiasis.
Hypoalbuminemia and conditions predisposing to its occurrence.
Systemic osteoporosis, myasthenia gravis, acute psychosis, poliomyelitis (except for the form of bulbar encephalitis), open- and closed-angle glaucoma.
Use of the drug in elderly patients (due to the high risk of developing osteoporosis and arterial hypertension).
Acute psychosis, severe affective disorders (including a history).
Eye infection due to herpes simplex virus (due to risk of corneal perforation).
During pregnancy.
In children during the growth period, GCS should be used only according to absolute indications and under especially careful supervision of the attending physician.
Side Effects
Side Effects
The incidence and severity of side effects depend on the duration of use, the size of the dose used and the ability to comply with the circadian rhythm of the prescription. Dexamethasone is usually well tolerated.
It has low mineralocorticoid activity, that is, its effect on water and electrolyte metabolism is small. As a rule, low and medium doses of dexamethasone do not cause sodium and water retention in the body or increased potassium excretion.
The following side effects have been reported:
Endocrine system disorders: decreased glucose tolerance, “steroidal” diabetes mellitus or manifestation of latent diabetes mellitus, suppressed adrenal function, Itsenko-Cushing syndrome (moon-shaped face, pituitary-type obesity, hirsutism, increased blood pressure, dysmenorrhea, amenorrhea, myasthenia gravis, striae), delayed sexual development in children.
Blood and lymphatic system disorders: moderate leukocytosis, leukocyturia, lymphopenia, eosinopenia, polycythemia.
Gastrointestinal tract disorders: nausea, vomiting, abdominal pain, discomfort in the epigastric region, pancreatitis, “steroid” gastric and duodenal ulcers, erosive esophagitis, bleeding and perforation of the stomach and intestinal wall, increased or decreased appetite, flatulence, hiccups. In rare cases, there is an increase in the activity of “liver” transaminases and alkaline phosphatase.
Disorders of the heart and blood vessels: arrhythmias, bradycardia (up to cardiac arrest); development (in predisposed patients) or progression of CHF, electrocardiographic changes characteristic of hypokalemia, increased blood pressure, hypercoagulation, thrombosis and thromboembolism, vasculitis, increased capillary fragility. In patients with acute and subacute myocardial infarction, the necrosis focus spreads, the formation of scar tissue slows down, which can lead to rupture of the heart muscle.
Nervous system disorders: increased intracranial pressure, cerebellar pseudotumor, headache, convulsions.
Mental disorders: nervousness or anxiety, insomnia, emotional lability, delirium, disorientation, euphoria, hallucinations, manic-depressive psychosis, depression, paranoia, suicidal tendencies.
Visual disorders: posterior subcapsular cataract, increased intraocular pressure with possible damage to the optic nerve, tendency to develop secondary bacterial, fungal or viral eye infections, trophic changes in the cornea, exophthalmos, corneal perforation, central serous chorioretinopathy.
Hearing and labyrinthine disorders: dizziness, vertigo.
Metabolic and nutritional disorders: hypercholesterolemia, increased excretion of calcium ions, hypocalcemia, weight gain, negative nitrogen balance (increased protein breakdown), increased sweating, epidural lipomatosis.
Caused by mineralocorticosteroid activity – fluid and sodium ion retention (peripheral edema), hypernatremia, hypokalemic syndrome (hypokalemia, arrhythmia, myalgia or muscle spasm, unusual weakness and fatigue).
Disorders of the musculoskeletal and connective tissue: slowing of growth and ossification processes in children (premature closure of the epiphyseal growth plates), osteoporosis (very rarely – pathological bone fractures, aseptic necrosis of the head of the humerus and femur), rupture of muscle tendons, “steroid” myopathy, decrease in muscle mass (atrophy).
Disorders of the skin and subcutaneous tissues: delayed wound healing, petechiae, ecchymoses, thinning of the skin, atrophy of the skin and subcutaneous tissue, hyper- or hypopigmentation, “steroid” acne, stretch marks, tendency to develop pyoderma and candidiasis, impaired skin pigmentation (hypo- or hyperpigmentation), telangiectasia.
Immune system disorders: generalized (skin rash, skin itching, anaphylactic shock), local allergic reactions.
Infectious and parasitic diseases: development or exacerbation of infections (the appearance of this side effect is facilitated by jointly used immunosuppressants and vaccination), masking of infections.
General disorders and disorders at the injection site: withdrawal syndrome.
Interaction
Interaction
Dexamethasone increases the toxicity of cardiac glycosides (due to the resulting hypokalemia, the risk of developing arrhythmias increases).
Accelerates the elimination of acetylsalicylic acid, reduces its concentration in the blood (when dexamethasone is discontinued, the concentration of salicylates in the blood increases and the risk of side effects increases).
When used simultaneously with live antiviral vaccines and against the background of other types of immunization, it increases the risk of viral activation and the development of infections.
Increases the metabolism of isoniazid, mexiletine (especially in “fast acetylators”), which leads to a decrease in their plasma concentrations.
Increases the risk of developing hepatotoxic effects of paracetamol (induction of “liver” enzymes and the formation of a toxic metabolite of paracetamol).
Increases (with long-term therapy) the content of folic acid.
Hypokalemia caused by GCS can increase the severity and duration of muscle blockade due to muscle relaxants.
In high doses, it reduces the effect of somatropin.
Antacids reduce the absorption of corticosteroids.
Dexamethasone reduces the effect of hypoglycemic drugs; enhances the anticoagulant effect of coumarin derivatives.
Weakens the effect of vitamin D on the absorption of calcium ions in the intestinal lumen. Ergocalciferol and parathyroid hormone prevent the development of osteopathy caused by GCS.
Reduces the concentration of praziquantel in the blood.
Cyclosporine (inhibits metabolism) and ketoconazole (reduces clearance) increase toxicity.
Thiazide diuretics, carbonic anhydrase inhibitors, other corticosteroids and amphotericin B increase the risk of hypokalemia, sodium-containing drugs – edema and increased blood pressure.
Non-steroidal anti-inflammatory drugs and ethanol increase the risk of developing ulceration of the gastrointestinal mucosa and bleeding; in combination with non-steroidal anti-inflammatory drugs for the treatment of arthritis, it is possible to reduce the dose of GCS due to the summation of the therapeutic effect.
Indomethacin, displacing dexamethasone from its connection with albumin, increases the risk of developing its side effects.
Amphotericin B and carbonic anhydrase inhibitors increase the risk of osteoporosis.
The therapeutic effect of GCS is reduced under the influence of phenytoin, barbiturates, ephedrine, theophylline, rifampicin and other inducers of “liver” microsomal enzymes (increased metabolic rate).
Mitotane and other inhibitors of adrenal function may necessitate an increase in the dose of GCS.
GCS clearance increases against the background of thyroid hormones.
Immunosuppressants increase the risk of developing infections and lymphoma or other lymphoproliferative disorders associated with Epstein-Barr virus.
Estrogens (including oral estrogen-containing contraceptives) reduce the clearance of corticosteroids, prolong the half-life and their therapeutic and toxic effects.
The appearance of hirsutism and acne is facilitated by the simultaneous use of other steroid hormonal drugs – androgens, estrogens, anabolic steroids, oral contraceptives.
Tricyclic antidepressants may increase the severity of depression caused by taking dexamethasone (not indicated for the treatment of these side effects).
The risk of developing cataracts increases when used in conjunction with other corticosteroids, antipsychotic drugs (neuroleptics), carbutamide and azathioprine.
Simultaneous administration with m-anticholinergic blockers (including antihistamines, tricyclic antidepressants), nitrates contributes to the development of increased intraocular pressure.
When used simultaneously with fluoroquinolones, the risk of tendonopathy (mainly Achilles tendon) increases in elderly patients and in patients with tendon diseases.
Antimalarials (chloroquine, hydroxychloroquine, mefloquine) in combination with dexamethasone may increase the risk of developing myopathy and cardiomyopathy.
Angiotensin-converting enzyme inhibitors, when administered concomitantly with dexamethasone, can change the composition of peripheral blood.
Overdose
Overdose
There may be an increase in dose-dependent side effects, with the exception of allergic reactions. It is necessary to reduce the dose of dexamethasone.
Treatment: symptomatic.
Storage conditions
Storage conditions
In a place protected from light at a temperature not exceeding 25 °C.
Keep out of the reach of children.
Shelf life
Shelf life
4 years.
Manufacturer
Manufacturer
Update of PFC JSC, Russia
Additional information
| Shelf life | 4 years. |
|---|---|
| Conditions of storage | In the dark place at a temperature no higher than 25 ° C. Keep out of reach of children. |
| Manufacturer | Update PFC AO, Russia |
| Medication form | pills |
| Brand | Update PFC AO |
Other forms…
Related products
Buy Dexamethasone, tablets 0.5 mg 56 pcs with delivery to USA, UK, Europe and over 120 other countries.