No products in the cart.
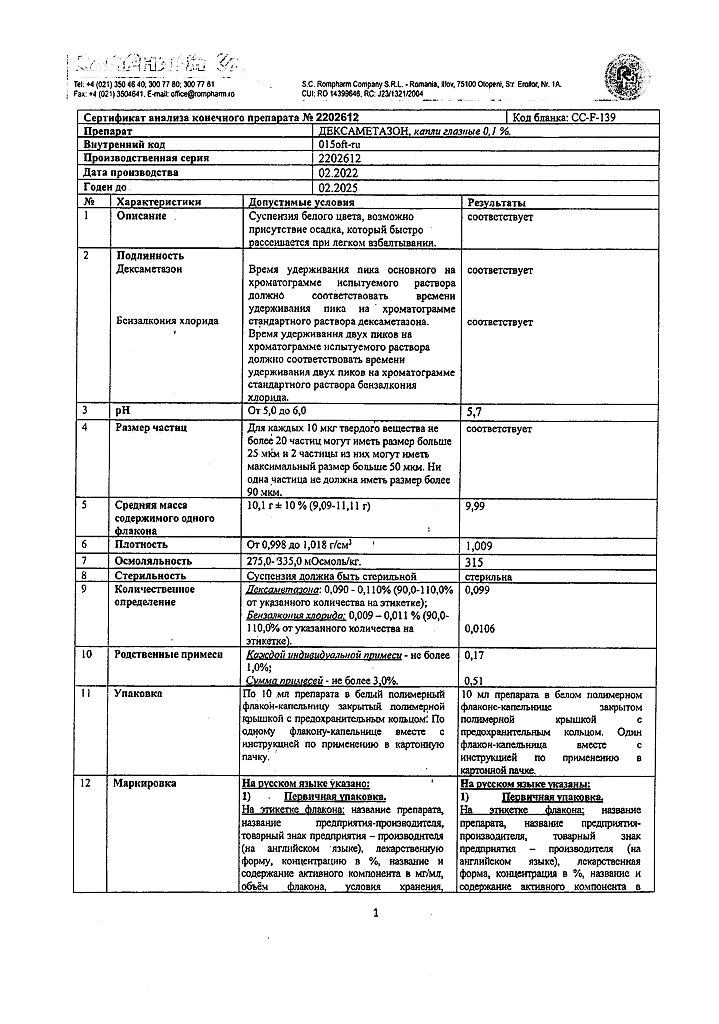
Dexamethasone, eye drops 0.1% 10 ml
€6.61 €5.51
Description
Dexamethasone is a glucocorticoid drug for topical use in ophthalmology. It has anti-inflammatory, anti-allergic and anti-exudative effects.
In the target tissues it interacts with specific protein receptors, regulates the expression of corticoid-dependent genes and thus affects protein synthesis. It decreases formation, release and activity of inflammatory mediators (including histamine, kininin, prostaglandins, lysosomal enzymes).
Inhibits cell migration to the site of inflammation. It reduces vasodilatation and increased vascular permeability in the inflammation area. It stabilizes the lysosomal enzymes of the leukocyte membranes. Inhibits antibody synthesis and disrupts antigen recognition.
Inhibits the release of interleukin 1 and interleukin 2, γ-interferon from lymphocytes and macrophages. Induces the formation of lipocortin, inhibits the release of inflammatory mediators by eosinophils and stabilizes the membranes of mast cells. All these effects are involved in suppressing the inflammatory reaction in tissues in response to mechanical, chemical or immune damage.
The duration of the anti-inflammatory effect of the drug after 1 drop of the solution is 4 to 8 hours.
Pharmacokinetics
After injection into the conjunctival sac, dexamethasone penetrates well into the corneal epithelium and conjunctiva, with therapeutic concentrations achieved in the aqueous humor of the eye. When the mucous membrane is inflamed or damaged, the rate of penetration increases.
The systemic absorption of the drug is minimal. About 60-70% of dexamethasone entering the systemic bloodstream is bound to plasma proteins.
Dexamethasone is metabolized in the liver under the action of cytochrome-containing enzymes. Metabolites are excreted with the feces. T1/2 averages 3 h.
Indications
Indications
Acute and chronic allergic and inflammatory diseases of the eye (including conjunctivitis, scleritis, deep keratitis without damage to the epithelium, iritis, iridocyclitis, choroiditis, chorioretinitis, optic neuritis), uveitis, episcleritis, blepharitis, scleritis, prevention and treatment of inflammatory processes in the postoperative and post-traumatic period, restoration of transparency corneas and decreased neovascularization after keratitis and burns.
Pharmacological effect
Pharmacological effect
Dexamethasone is a glucocorticoid drug for topical use in ophthalmology. It has anti-inflammatory, anti-allergic and anti-exudative effects.
In target tissues, it interacts with specific protein receptors, regulates the expression of corticoid-dependent genes and thus affects protein synthesis. Reduces the formation, release and activity of inflammatory mediators (including histamine, kinin, prostaglandins, lysosomal enzymes).
Suppresses cell migration to the site of inflammation. Reduces vasodilation and increased vascular permeability at the site of inflammation. Stabilizes lysosomal enzymes of leukocyte membranes. Suppresses antibody synthesis and disrupts antigen recognition.
Inhibits the release of interleukin 1 and interleukin 2, γ-interferon from lymphocytes and macrophages. Induces the formation of lipocortin, inhibits the release of inflammatory mediators by eosinophils and stabilizes mast cell membranes. All of these effects are involved in suppressing the inflammatory response in tissues in response to mechanical, chemical or immune damage.
The duration of the anti-inflammatory effect of the drug after instillation of 1 drop of solution is from 4 to 8 hours.
Pharmacokinetics
After instillation into the conjunctival sac, dexamethasone penetrates well into the corneal epithelium and conjunctiva, and therapeutic concentrations are achieved in the aqueous humor of the eye. When the mucous membrane is inflamed or damaged, the penetration rate increases.
Systemic absorption of the drug is minimal. About 60-70% of dexamethasone entering the systemic circulation is bound to plasma proteins.
Dexamethasone is metabolized in the liver under the action of cytochrome-containing enzymes. Metabolites are excreted in feces. T1/2 averages 3 hours.
Special instructions
Special instructions
The drug should not be used for intraocular injection.
Intended for topical use only.
The drug should be shaken before direct use.
If there is no improvement after 3-4 days of treatment, additional local or systemic treatment should be prescribed.
Do not use the drug while wearing soft contact lenses. The drug contains the antimicrobial preservative benzalkonium chloride, which can be adsorbed by soft contact lenses. It is necessary to remove the lenses before using the drug and install them no earlier than 15 minutes after instillation.
When using the drug in combination with other ophthalmic drops, it is necessary to maintain an interval between instillations of at least 15 minutes.
Treatment with the drug may mask the picture of a bacterial or fungal infection, therefore, when treating infectious eye diseases, the drug should be combined with adequate antimicrobial therapy.
Dexamethasone may cause a false positive drug test result.
Impact on the ability to drive vehicles and operate machinery
Taking into account the possibility of lacrimation after instillation of the drug, it should not be used immediately before driving vehicles or working with machinery. Within 30 minutes after instillation of the drug, you must refrain from activities that require increased attention.
Active ingredient
Active ingredient
Dexamethasone
Composition
Composition
Active ingredient:
dexamethasone sodium phosphate 1 mg;
Excipients:
polysorbate 80,
hypromellose (hydroxypropyl methylcellulose 4000),
disodium phosphate dodecahydrate,
citric acid monohydrate,
sodium chloride,
disodium edetate dihydrate,
benzalkonium chloride,
purified water.
Pregnancy
Pregnancy
The use of the drug during pregnancy during lactation (breastfeeding) is contraindicated.
Use in children
Contraindication: children under 6 years of age.
Contraindications
Contraindications
viral eye diseases;
fungal eye diseases;
purulent eye diseases (without concomitant antimicrobial therapy);
trachoma;
increased intraocular pressure;
damage to the integrity of the corneal epithelium;
eye tuberculosis;
children under 6 years of age;
pregnancy;
lactation period (breastfeeding);
hypersensitivity to the components of the drug.
Side Effects
Side Effects
With short-term therapy: nausea, vomiting, bradycardia, arrhythmias, ulceration of the gastrointestinal mucosa, decreased immunity.
With long-term use: Itsenko-Cushing syndrome, hyperglycemia, pancreatitis, myocardial dystrophy, headache, convulsions, psychosis.
Local reactions: immediately after instillation of the drug, a quickly passing burning sensation is possible. With prolonged use, intraocular pressure may increase (therefore, when using drugs containing corticosteroids for more than 10 days, intraocular pressure should be measured regularly).
When used for more than 3 months, posterior capsular cataracts may develop. Regeneration processes may slow down.
Interaction
Interaction
Long-term use of dexamethasone with idoxuridine may increase destructive processes in the corneal epithelium.
Dexamethasone may enhance the effect of barbiturates.
The combined use of phenytoin with dexamethasone leads to a decrease in the concentration of the latter.
Warfarin combined with dexamethasone increases the risk of bleeding.
In the usual regimen of topical administration, the dose is insufficient to cause induction or saturation of liver enzymes.
With simultaneous use of dexamethasone with diuretics (especially thiazide and carbonic anhydrase inhibitors) and amphotericin B, it is possible to increase the excretion of potassium from the body and increase the risk of developing heart failure.
With the simultaneous use of dexamethasone with cardiac glycosides, their tolerability worsens and the likelihood of developing ventricular extrasystole (due to hypokalemia) increases.
With the simultaneous use of dexamethasone with ethanol and NSAIDs, the risk of erosive and ulcerative lesions of the gastrointestinal tract increases.
Estrogens and oral contraceptives containing estrogen reduce the clearance of dexamethasone, which may be accompanied by an increase in the severity of its action.
The combined use of antiarrhythmic drugs with dexamethasone may lead to a decrease in the effect of the latter.
Overdose
Overdose
Overdose when used in ophthalmology is unlikely.
Symptoms: local manifestations are possible. There is no specific antidote. The drug should be discontinued and symptomatic therapy prescribed.
Long-term use of the drug or use in large doses may increase the systemic absorption of dexamethasone, and may also develop ocular hypertension and certain diseases of the cornea or lens.
Storage conditions
Storage conditions
The drug should be stored in a place protected from light, out of reach of children, at a temperature of 15° to 25°C.
Shelf life
Shelf life
3 years. After opening the bottle, the drug should be stored for no more than 4 weeks.
Manufacturer
Manufacturer
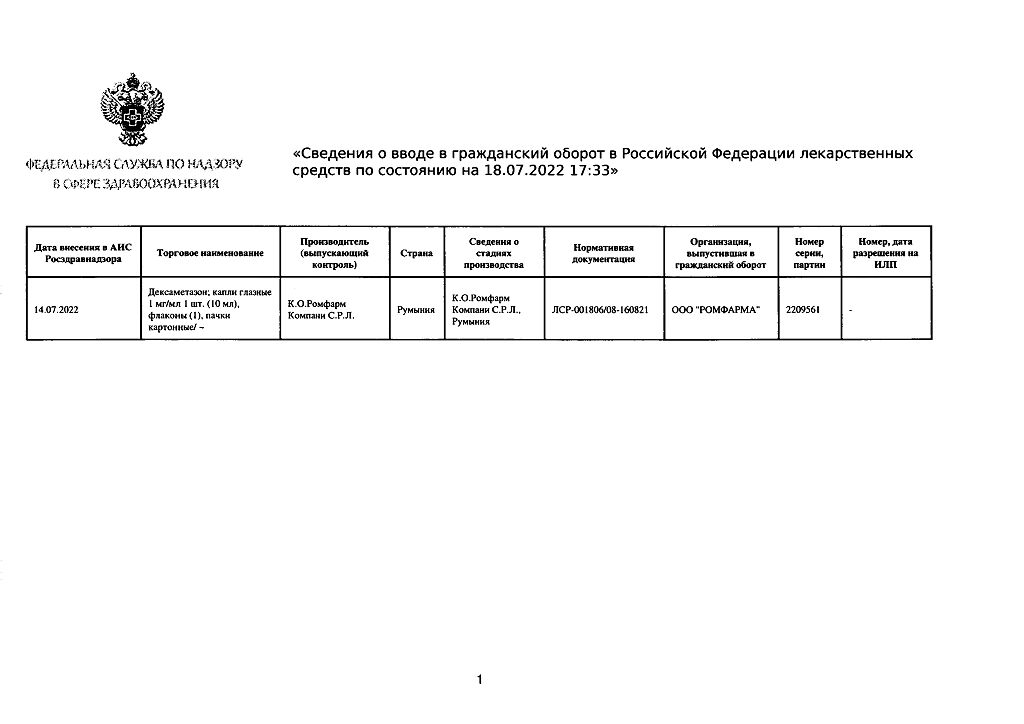
K.O.Rompharm Company S.R.L., Romania
Additional information
| Shelf life | 3 years. After opening the bottle the drug should not be stored more than 4 weeks. |
|---|---|
| Conditions of storage | The drug should be stored in the dark place out of the reach of children at 15° to 25°C. |
| Manufacturer | C.O.Rompharm Company S.R.L., Romania |
| Medication form | eye drops |
| Brand | C.O.Rompharm Company S.R.L. |
Other forms…
Related products
Buy Dexamethasone, eye drops 0.1% 10 ml with delivery to USA, UK, Europe and over 120 other countries.