No products in the cart.
Description
Pharmgroup: anti-allergic agent – H1-histamine receptor blocker.
Pharmaceutical effect: Blocker of H1-histamine receptors (long-acting). It is the primary active metabolite of loratadine.
Suppresses the release of histamine and leukotriene C4 from mast cells.
Prevents the development and facilitates the course of allergic reactions.
It has anti-allergic, antipruritic and antiexudative action.
Limits capillary permeability, prevents development of tissue edema, relieves smooth muscle spasm.
It has practically no sedative effect and when taken at a dose of 7.5 mg it does not affect the speed of psychomotor reactions.
In comparative studies of desloratadine and loratadine no qualitative or quantitative differences in toxicity of the 2 drugs in comparable doses (taking into account desloratadine concentration) were found.
Indications
Indications
Seasonal and year-round allergic rhinitis, chronic idiopathic urticaria.
Pharmacological effect
Pharmacological effect
Pharmaceutical group: antiallergic agent – H1-histamine receptor blocker.
Pharmaceutical action: H1-histamine receptor blocker (long-acting). It is the primary active metabolite of loratadine.
Suppresses the release of histamine and leukotriene C4 from mast cells.
Prevents the development and facilitates the course of allergic reactions.
It has antiallergic, antipruritic and antiexudative effects.
Reduces capillary permeability, prevents the development of tissue edema, relieves spasm of smooth muscles.
It has virtually no sedative effect and, when taken at a dose of 7.5 mg, does not affect the speed of psychomotor reactions.
In comparative studies of desloratadine and loratadine, no qualitative or quantitative differences in the toxicity of 2 drugs in comparable doses (taking into account the concentration of desloratadine) were revealed.
Special instructions
Special instructions
There have been no studies of the effectiveness of desloratadine in rhinitis of infectious etiology.
Impact on the ability to drive vehicles and machinery
When taking desloratadine at the recommended dose, no adverse effects on driving vehicles or using machinery were observed.
However, in rare cases, some patients experience drowsiness and dizziness while taking the drug, which may affect the ability to drive vehicles and operate machines.
Active ingredient
Active ingredient
Desloratadine
Composition
Composition
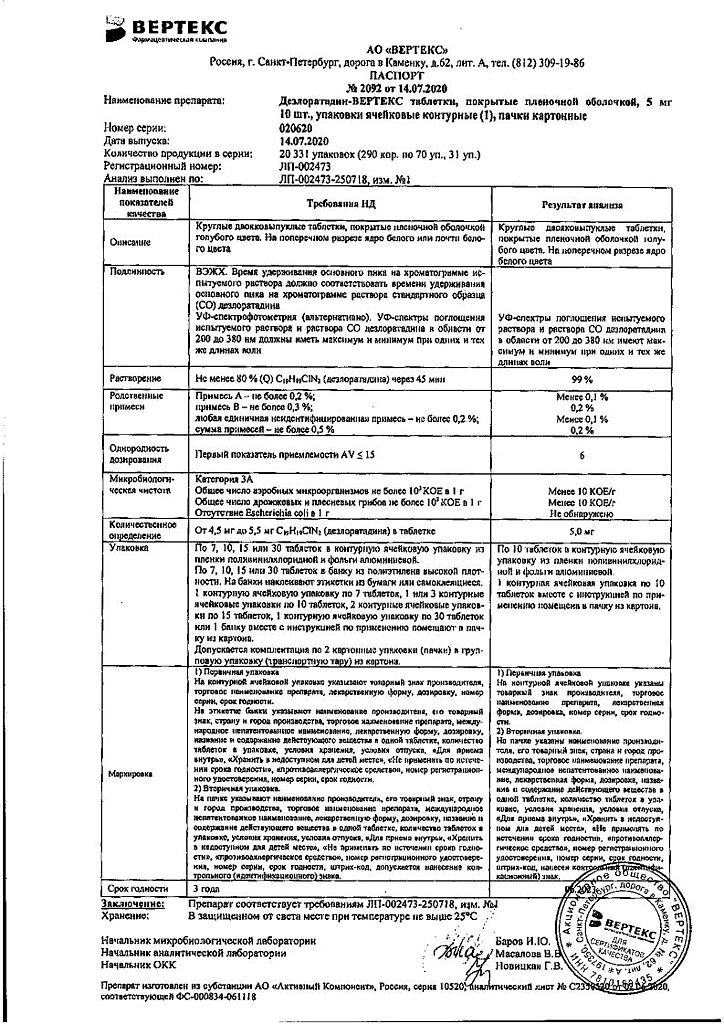
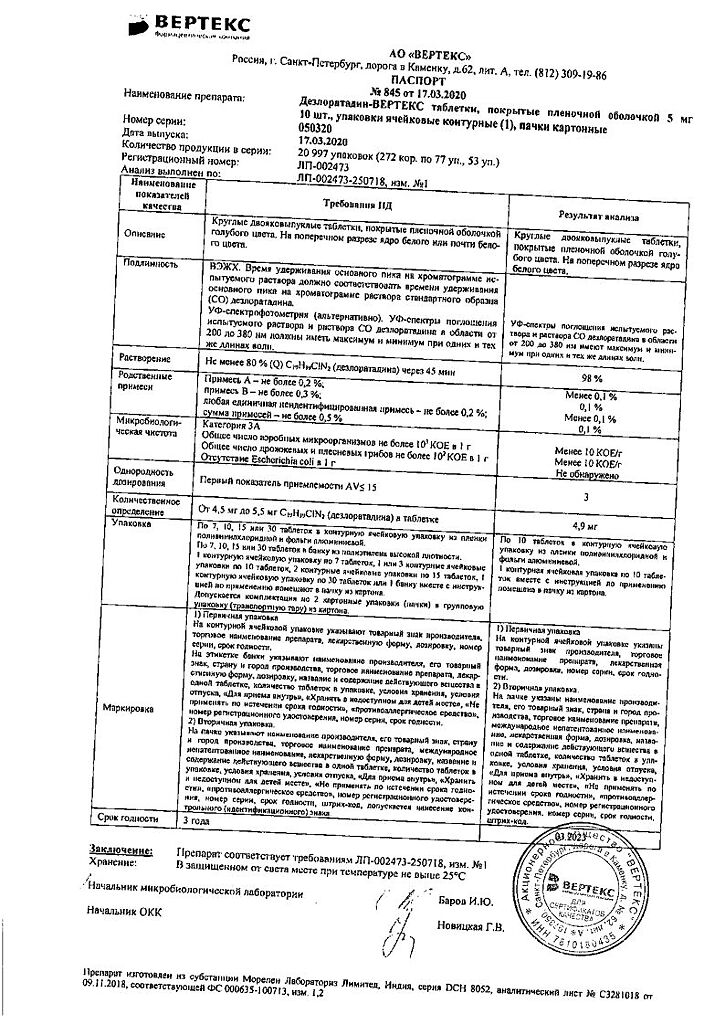
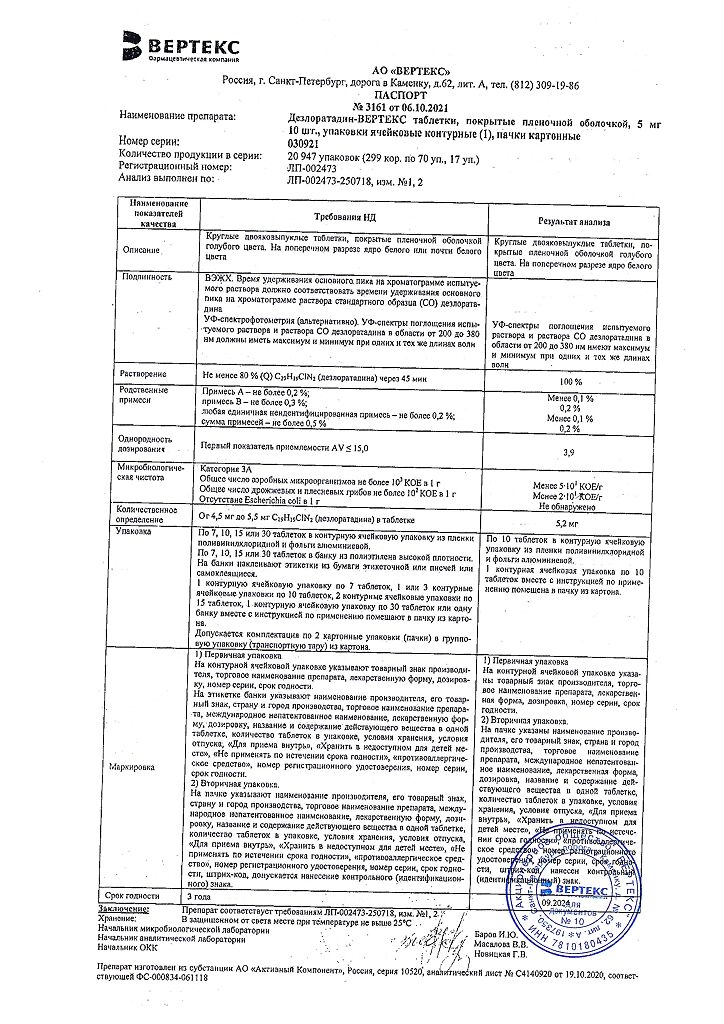
Blue film-coated tablets, round, biconvex; On a cross section, the core is white or almost white.
1 tablet: desloratadine 5 mg
Excipients:
calcium hydrogen phosphate dihydrate – 53 mg,
microcrystalline cellulose – 27.5 mg,
corn starch – 11 mg,
talc – 2.5 mg,
magnesium stearate – 1 mg.
Film shell composition:
dry mixture for film coating (polyvinyl alcohol – 40%, titanium dioxide – 22.1%, macragol 3350 (polyethylene glycol 3350) – 20.2%, talc – 14.8%, aluminum varnish based on indigo carmine dye – 2.8%, yellow iron oxide (iron oxide) – 0.1%) – 3 mg.
Pregnancy
Pregnancy
The use of the drug during pregnancy is not recommended. Since cetirizine passes into breast milk, it is not prescribed during breastfeeding.
Contraindications
Contraindications
Hypersensitivity, pregnancy, lactation period, children’s age (up to 1 year), children’s age (up to 12 years) for tablet forms.
For syrup (additionally, due to the presence of sucrose and sorbitol): hereditary fructose intolerance, glucose/galactose malabsorption or sucrose/isomaltose deficiency.
With caution. Severe renal failure.
Side Effects
Side Effects
Rarely: dizziness, drowsiness, tachycardia, palpitations, abdominal pain, dyspepsia (including nausea, vomiting, diarrhea), hyperbilirubinemia, increased activity of liver enzymes, allergic reactions (skin rash, itching, urticaria, angioedema, anaphylactic shock).
In children under 2 years of age (frequency slightly higher than when taking placebo): diarrhea, hyperthermia, insomnia.
In 2-11 year olds, the incidence of side effects is comparable to placebo.
In adults and children over 12 years of age (the frequency is slightly higher than when taking placebo): increased fatigue, dry mouth, headache; drowsiness (incidence comparable to placebo).
Interaction
Interaction
Interactions with other drugs were not detected in studies with azithromycin and ketoconazole. erythromycin, fluoxetine and cimetidine.
Eating does not affect the effectiveness of the drug.
Desloratadine does not enhance the effects of alcohol on the central nervous system.
Overdose
Overdose
Symptoms
Taking a dose 5 times the recommended dose did not result in any symptoms. Daily use of desloratadine in adults and adolescents at a dose of up to 20 mg for 14 days was not associated with statistically or clinically significant changes in the cardiovascular system. The use of desloratadine at a dose of 45 mg per day (9 times higher than recommended) for 10 days did not cause prolongation of the QT interval and was not accompanied by the occurrence of serious side effects.
Treatment
If you accidentally ingest a large amount of the drug, you should immediately consult a doctor. It is recommended to lavage the stomach, take activated charcoal, and, if necessary, symptomatic therapy. Desloratadine is not excreted by hemodialysis; the effectiveness of hypertensive dialysis has not been established.
Storage conditions
Storage conditions
In a dry place, protected from light, at a temperature not exceeding 25 ° C. Keep out of the reach of children.
Shelf life
Shelf life
2 years. Do not use after expiration date.
Manufacturer
Manufacturer
Vertex, Russia
Additional information
| Shelf life | 2 years. Do not use after the expiration date. |
|---|---|
| Conditions of storage | Store in a dry place protected from light at a temperature not exceeding 25 °С. Keep out of reach of children. |
| Manufacturer | Vertex, Russia |
| Medication form | pills |
| Brand | Vertex |
Other forms…
Related products
Buy Desloratadin-Vertex, 5 mg 10 pcs with delivery to USA, UK, Europe and over 120 other countries.