No products in the cart.
Cycloferon, 125 mg/ml 2 ml 5 pcs
€10.94 €9.57
Description
Pharmacotherapeutic group: other immunostimulants.
ATX code: L03AX.
Pharmacological properties
Pharmacodynamics
CYCLOPHERON® is a low molecular weight interferon inducer, which determines a wide range of its biological activity (antiviral, immunomodulatory, anti-inflammatory, etc.).
The main interferon-producing cells after administration of CYCLOPHERON® preparation are macrophages, T- and B-lymphocytes. Depending on the type of infection there is a predominance of activity of one or another link of immunity. The drug induces high titers of interferon in organs and tissues containing lymphoid elements (spleen, liver, lungs), activates stem cells of bone marrow, stimulating the formation of granulocytes. CYCLOPHERON® activates T-lymphocytes and natural killer cells, normalizes the balance between subpopulations of T-helpers and T-suppressors. It increases activity of α-interferons.
CYCLOPHERON® shows antiviral activity against tick-borne encephalitis, influenza, herpes, cytomegalovirus, papilloma virus and other viruses. Preclinical studies have shown that the use of Cycloferon in the early stages of the infection process leads to a decrease in the viral load (virus titer) in lung tissues. It increases nonspecific resistance of the body against viral and bacterial infections.
The high effectiveness of the drug was found in the treatment of acute and chronic bacterial infections (neuroinfections, chlamydia) as a component of immunotherapy.
Pharmacokinetics
When administered the maximum allowable dose the maximum concentration in blood is reached after 1-2 hours, after 24 hours the drug is detected in trace amounts. It crosses the blood-brain barrier. Half-life period of the drug is 4-5 hours. CYCLOPHERON® has no cumulative properties. It does not accumulate in the tissues after prolonged use.
Indications
Indications
In adults in complex therapy:
– herpes and cytomegalovirus infection;
– neuroinfections: serous meningitis and encephalitis, tick-borne borreliosis (Lyme disease);
– secondary immunodeficiencies associated with acute and chronic bacterial and fungal infections;
– chlamydial infections.
In children over 4 years of age in complex therapy:
– in complex therapy of herpetic infection.
Pharmacological effect
Pharmacological effect
Pharmacotherapeutic group: other immunostimulants.
ATX code: L03AX.
PHARMACOLOGICAL PROPERTIES
Pharmacodynamics
CYCLOFERON® is a low-molecular-weight interferon inducer, which determines a wide range of its biological activity (antiviral, immunomodulatory, anti-inflammatory, etc.).
The main interferon-producing cells after administration of the drug CYCLOFERON® are macrophages, T- and B-lymphocytes. Depending on the type of infection, the activity of one or another part of the immune system predominates. The drug induces high titers of interferon in organs and tissues containing lymphoid elements (spleen, liver, lungs), activates bone marrow stem cells, stimulating the formation of granulocytes. CYCLOFERON® activates T-lymphocytes and natural killer cells, normalizes the balance between subpopulations of T-helpers and T-suppressors. Enhances the activity of α-interferons.
CYCLOFERON® demonstrates antiviral activity against pathogens of tick-borne encephalitis, influenza, herpes, cytomegalovirus, papilloma virus and other viruses. Preclinical studies have shown that the use of Cycloferon in the early stages of the infectious process leads to a decrease in the viral load (virus titer) in lung tissue. Increases the body’s nonspecific resistance to viral and bacterial infections.
The drug has been found to be highly effective in complex therapy of acute and chronic bacterial infections (neuroinfections, chlamydia) as a component of immunotherapy.
Pharmacokinetics
When the maximum permissible dose is administered, the maximum concentration in the blood is achieved after 1–2 hours; after 24 hours the drug is detected in trace amounts. Crosses the blood-brain barrier. The half-life is 4–5 hours. CYCLOFERON® does not have cumulative properties. Does not accumulate in tissues with prolonged use.
Special instructions
Special instructions
For diseases of the thyroid gland, consultation with an endocrinologist is necessary. Urine may be colored violet-blue (luminescence). If the color of the solution changes and a precipitate forms, the use of the drug is unacceptable.
Effect on the ability to drive vehicles and machinery CYCLOFERON® does not affect the ability to drive vehicles or machinery.
Active ingredient
Active ingredient
Meglumine acridone acetate
Composition
Composition
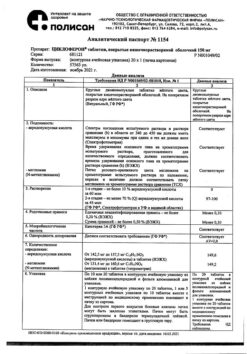
Active ingredient:
Meglumine acridone acetate in terms of acridone acetic acid 125.0 mg, obtained according to the following recipe: Acridone acetic acid 125.0 mg Meglumine (N-methylglucamine) 96.3 mg
Excipient: Water for injection up to 1.0 ml
Pregnancy
Pregnancy
The use of the drug during pregnancy and breastfeeding is contraindicated.
Contraindications
Contraindications
Pregnancy, breastfeeding, hypersensitivity to the components of the drug, liver cirrhosis in the stage of decompensation, children under 18 years of age (for all indications, with the exception of the indication “in the complex therapy of herpetic infection”, children under 4 years of age (for the indication “in the complex therapy of herpetic infection”).
Side Effects
Side Effects
According to the World Health Organization, undesirable effects are classified according to the frequency of their development as follows:
– very frequent (≥ 1/10);
– frequent (≥ 1/100 – < 1/10);
– uncommon (≥ 1/1000 – < 1/100);
– rare (≥ 1/10000 – < 1/1000);
– very rare (< 1/10000);
– frequency unknown (cannot be determined based on available data).
General disorders and disorders at the injection site: very rarely – chills, fever, pain and redness at the injection site. Disorders of the skin and subcutaneous tissues: very rarely – rash, urticaria. If any of the undesirable effects indicated in the instructions worsen or you notice any other undesirable effects not listed in the instructions, tell your doctor.
Interaction
Interaction
CYCLOFERON® is compatible and goes well with all drugs traditionally used in the treatment of these diseases (interferons, chemotherapeutic drugs, etc.). Enhances the effect of interferons and nucleoside analogues. Reduces the side effects of chemotherapy, interferon therapy.
Storage conditions
Storage conditions
In a place protected from light at a temperature of 0 to 25 ºС. Keep out of the reach of children.
Shelf life
Shelf life
5 years. After the expiration date, the use of the drug is not allowed.
Manufacturer
Manufacturer
Polisan NTFF LLC, Russia
Additional information
| Shelf life | 5 years. After the expiration date the use of the drug is not allowed. |
|---|---|
| Conditions of storage | Store in a light-protected place at temperatures from 0 to 25 ºC. Keep out of reach of children. |
| Manufacturer | Polysan NTFF LLC, Russia |
| Medication form | solution |
| Brand | Polysan NTFF LLC |
Other forms…
Related products
Buy Cycloferon, 125 mg/ml 2 ml 5 pcs with delivery to USA, UK, Europe and over 120 other countries.