No products in the cart.
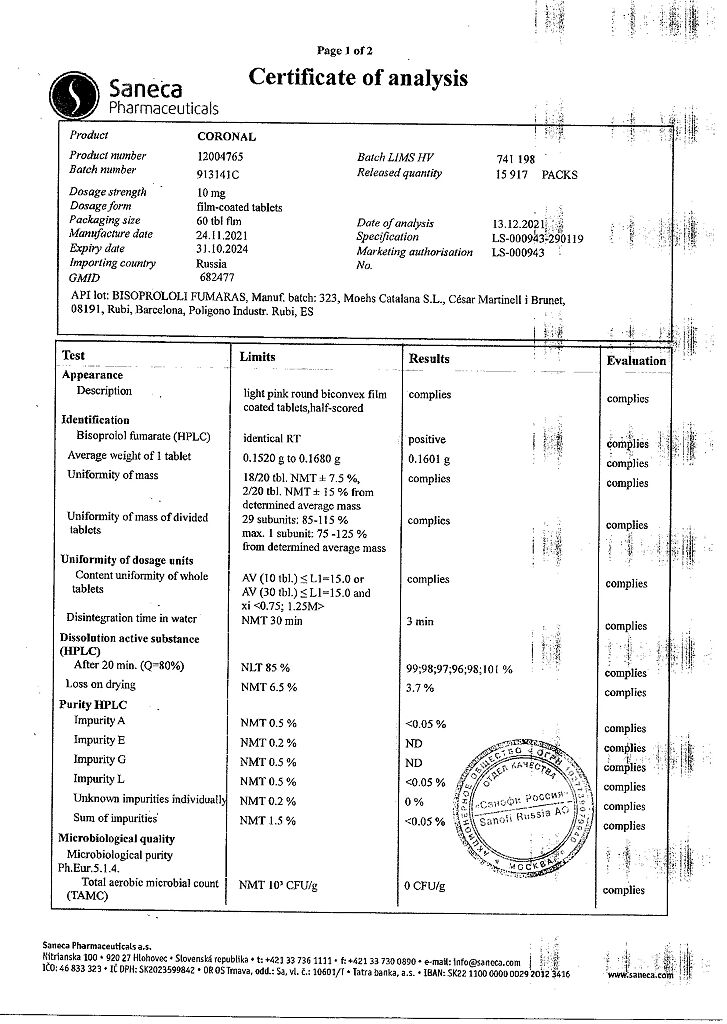
Coronal, 10 mg 60 pcs
€8.84 €7.74
Description
Coronal is a selective beta1-adrenoblocker without sympathomimetic activity of its own. It has no membrane stabilizing effect. It reduces plasma renin activity, decreases myocardial oxygen demand, decreases HR at rest and under load. It has hypotensive, antiarrhythmic and anti-anginal action.
Hypotensive action is associated with the reduction of the blood minute volume, sympathetic stimulation of peripheral vessels, decrease of renin-angiotensin system activity (it is more important for patients with initial renin hypersecretion), restoration of sensitivity in response to lowering blood pressure and the effect on CNS. In arterial hypertension hypotensive effect develops in 2-5 days, stable effect – in 1-2 months.
Antianginal action is due to decrease of myocardial oxygen demand as a result of slow heart rate and decreased contractility, prolongation of diastole, improvement of myocardial perfusion. Due to increased end diastolic pressure in the left ventricle and increased stretching of ventricular muscle fibers, oxygen demand may increase, especially in patients with chronic heart failure.
Antiarrhythmic action is caused by the removal of arrhythmogenic factors (tachycardia, increased activity of the sympathetic nervous system, increased content of CAMF, arterial hypertension), the decrease of spontaneous excitation rate of sinus and ectopic pacemakers and AV conduction slowing (mainly in antegrade and, to smaller extent, in retrograde direction via AV node) and conduction via additional pathways. When used in medium therapeutic doses, unlike non-selective beta-adrenoblockers, it has less pronounced effect on the organs containing β2-adrenoreceptors (pancreas, skeletal muscles, smooth muscles of peripheral arteries, bronchi and uterus) and on carbohydrate metabolism, does not cause retention of sodium ions in the body. When used in high doses (200 mg and more) it has a blocking effect on both subtypes of β-adrenoreceptors, mainly in the bronchi and vascular smooth muscle.
Indications
Indications
— arterial hypertension;
— IHD: prevention of angina attacks.
Pharmacological effect
Pharmacological effect
Coronal is a selective beta1-blocker without its own sympathomimetic activity. Does not have a membrane stabilizing effect. Reduces plasma renin activity, reduces myocardial oxygen demand, reduces heart rate at rest and during exercise. Has hypotensive, antiarrhythmic and antianginal effects.
The hypotensive effect is associated with a decrease in minute blood volume, sympathetic stimulation of peripheral vessels, a decrease in the activity of the renin-angiotensin system (of greater importance for patients with initial hypersecretion of renin), restoration of sensitivity in response to a decrease in blood pressure and an effect on the central nervous system. In arterial hypertension, the hypotensive effect develops after 2-5 days, a stable effect – after 1-2 months.
The antianginal effect is due to a decrease in myocardial oxygen demand as a result of a decrease in heart rate and decreased contractility, prolongation of diastole, and improved myocardial perfusion. Due to increased end-diastolic pressure in the left ventricle and increased stretch of ventricular muscle fibers, oxygen demand may increase, especially in patients with chronic heart failure.
The antiarrhythmic effect is due to the elimination of arrhythmogenic factors (tachycardia, increased activity of the sympathetic nervous system, increased cAMP content, arterial hypertension), a decrease in the rate of spontaneous excitation of sinus and ectopic pacemakers and a slowdown in AV conduction (mainly in the antegrade and, to a lesser extent, in the retrograde directions through the AV node) and conduction along additional pathways. When used in average therapeutic doses, in contrast to non-selective beta-blockers, it has a less pronounced effect on organs containing β2-adrenergic receptors (pancreas, skeletal muscles, smooth muscles of peripheral arteries, bronchi and uterus) and on carbohydrate metabolism, does not cause sodium ion retention in the body. When used in high doses (200 mg or more), it has a blocking effect on both subtypes of β-adrenergic receptors, mainly in the bronchi and vascular smooth muscles.
Special instructions
Special instructions
When prescribing Coronal, you should regularly monitor heart rate and blood pressure (at the beginning of treatment – daily, then once every 3-4 months), perform an ECG, and determine the blood glucose level in patients with diabetes (once every 4-5 months). In elderly patients, it is recommended to monitor renal function (once every 4-5 months).
The patient should be trained in the method of calculating heart rate and instructed about the need for medical consultation if the heart rate is less than 50 beats/min.
Before starting treatment, it is recommended to conduct a study of external respiratory function in patients with a burdened bronchopulmonary history.
It should be taken into account that in approximately 20% of patients with angina, beta-blockers are ineffective due to severe coronary atherosclerosis with a low ischemic threshold (heart rate less than 100 beats/min) and increased end-diastolic volume of the left ventricle, which impairs subendocardial blood flow.
In smoking patients, the effectiveness of beta-blockers is reduced.
Patients using contact lenses should take into account that during treatment the production of tear fluid may decrease.
When using Coronal in patients with pheochromocytoma, there is a risk of developing paradoxical arterial hypertension (if effective alpha-blockade is not previously achieved).
Bisoprolol may mask certain clinical signs of thyrotoxicosis (eg, tachycardia). Abrupt withdrawal of Coronal in patients with thyrotoxicosis is contraindicated, since it can increase the symptoms of the disease.
In diabetes mellitus, bisoprolol can mask tachycardia caused by hypoglycemia. Unlike non-selective beta-blockers, it practically does not enhance insulin-induced hypoglycemia and does not delay the restoration of blood glucose concentrations to normal levels.
When taking clonidine concomitantly, it can be discontinued only a few days after discontinuation of the drug Coronal.
It is possible that the severity of the hypersensitivity reaction may increase and there will be no effect from usual doses of epinephrine against the background of a burdened allergic history.
If planned surgical treatment is necessary, the drug should be discontinued 48 hours before the start of general anesthesia. If the patient took the drug before surgery, he should select a drug for general anesthesia with minimal negative inotropic effect.
Reciprocal activation of the vagus nerve can be eliminated by intravenous atropine (1-2 mg).
Medicines that reduce the supply of catecholamines (including reserpine) can enhance the effect of beta-blockers, so patients taking such combinations of drugs should be under constant medical supervision to detect a pronounced decrease in blood pressure or bradycardia.
Patients with concomitant bronchospastic diseases can be prescribed cardioselective adrenergic blockers in case of intolerance and/or ineffectiveness of other antihypertensive drugs. An overdose is dangerous due to the development of bronchospasm.
If increasing bradycardia (less than 50 beats/min), a pronounced decrease in blood pressure (systolic blood pressure below 100 mm Hg), or AV blockade occurs in elderly patients, it is necessary to reduce the dose or stop treatment.
It is recommended to discontinue therapy if depression develops.
The drug should be discontinued before testing the content of catecholamines, normetanephrine, vanillinmandelic acid, and antinuclear antibody titers in the blood and urine.
Treatment should not be abruptly interrupted due to the risk of developing severe arrhythmias and myocardial infarction. Cancellation is carried out gradually, reducing the dose over 2 weeks or more (the dose is reduced by 25% in 3-4 days).
Use in pediatrics
The use of the drug Coronal in children under 18 years of age is contraindicated, since the effectiveness and safety have not been established.
Impact on the ability to drive vehicles and operate machinery
During the treatment period, care must be taken when driving vehicles and engaging in other potentially hazardous activities that require increased concentration and speed of psychomotor reactions.
Active ingredient
Active ingredient
Bisoprolol
Composition
Composition
1 tablet contains
10 mg Bisoprolol fumarate;
Excipients:
microcrystalline cellulose,
corn starch,
sodium lauryl sulfate,
anhydrous colloidal silicon dioxide,
magnesium stearate.
Contraindications
Contraindications
— shock (including cardiogenic);
— collapse;
– pulmonary edema;
— acute heart failure;
— chronic heart failure in the stage of decompensation;
— AV blockade II and III degrees;
— sinoatrial blockade;
— SSSU;
– severe bradycardia;
— angiospastic angina (Prinzmetal angina);
— cardiomegaly (without signs of heart failure);
– arterial hypotension (systolic blood pressure below 100 mm Hg, especially with myocardial infarction);
– history of bronchial asthma and chronic obstructive pulmonary disease;
– simultaneous use of MAO inhibitors (with the exception of MAO type B inhibitors);
— late stages of peripheral circulatory disorders;
— Raynaud’s disease;
— pheochromocytoma (without simultaneous use of alpha-blockers);
— metabolic acidosis;
– age under 18 years (efficacy and safety have not been established);
– hypersensitivity to the components of the drug and other beta-blockers.
Side Effects
Side Effects
The frequency of occurrence of side effects is determined as follows: very often (≥1/10), often (≥1/100 and From the central nervous system and peripheral nervous system: infrequently – increased fatigue, asthenia, dizziness, headache, drowsiness or insomnia, depression; rarely – hallucinations, nightmares, convulsions.
From the senses: rarely – blurred vision, decreased secretion of tear fluid, dry and sore eyes, hearing impairment; very rarely – conjunctivitis.
From the cardiovascular system: very often – sinus bradycardia; often – decreased blood pressure, manifestation of vasospasm (increased peripheral circulatory disorders, coldness of the lower extremities, paresthesia); uncommon – impaired AV conduction, orthostatic hypotension, decompensation of chronic heart failure, peripheral edema.
From the digestive system: often – dryness of the oral mucosa, nausea, vomiting, diarrhea, constipation; rarely – hepatitis, increased activity of liver transaminases.
From the respiratory system: infrequently – difficulty breathing when prescribed in high doses (loss of selectivity) and/or in predisposed patients – laryngo- and bronchospasm; rarely – nasal congestion, allergic rhinitis.
From the endocrine system: rarely – hyperglycemia (in patients with type 2 diabetes mellitus), hypoglycemia (in patients receiving insulin).
Allergic reactions: rarely – itching, rash, urticaria.
Dermatological reactions: rarely – increased sweating, skin hyperemia; very rarely – psoriasis-like skin reactions, exacerbation of psoriasis symptoms, alopecia.
From the musculoskeletal system: infrequently – muscle weakness, cramps in the calf muscles, arthralgia.
From the hematopoietic system: in some cases – thrombocytopenia, agranulocytosis.
Other: rarely – hypertriglyceridemia; very rarely – impaired potency, rarely – withdrawal syndrome (increased angina attacks, increased blood pressure).
Interaction
Interaction
Allergens used for immunotherapy or allergen extracts for skin testing increase the risk of severe systemic allergic reactions or anaphylaxis in patients receiving bisoprolol.
When used simultaneously with Coronal, iodine-containing radiopaque drugs for intravenous administration increase the risk of developing anaphylactic reactions.
When used simultaneously with Coronal, phenytoin for intravenous administration and drugs for inhalation general anesthesia (hydrocarbon derivatives) increase the severity of the cardiodepressive effect and the likelihood of a decrease in blood pressure.
When used simultaneously, Coronal changes the effectiveness of insulin and oral hypoglycemic drugs, masks the symptoms of developing hypoglycemia (tachycardia, increased blood pressure).
With simultaneous use, Coronal reduces the clearance of lidocaine and xanthines (except diphylline) and increases their concentration in plasma, especially in patients with initially increased clearance of theophylline under the influence of smoking.
NSAIDs (due to the retention of sodium ions and blockade of prostaglandin synthesis by the kidneys), corticosteroids and estrogens (due to the retention of sodium ions) weaken the hypotensive effect of Coronal.
When used simultaneously with Coronal, cardiac glycosides, methyldopa, reserpine and guanfacine, slow calcium channel blockers (verapamil, diltiazem), amiodarone and other antiarrhythmic drugs increase the risk of developing or worsening bradycardia, AV block, cardiac arrest and heart failure.
When used simultaneously with Coronal, nifedipine can lead to a significant decrease in blood pressure.
When used simultaneously with Coronal, diuretics, clonidine, sympatholytics, hydralazine and other antihypertensive drugs can lead to an excessive decrease in blood pressure.
Coronal prolongs the action of non-depolarizing muscle relaxants and the anticoagulant effect of coumarins.
When used simultaneously with Coronal, tricyclic and tetracyclic antidepressants, antipsychotic drugs (neuroleptics), ethanol, sedatives and hypnotics increase CNS depression.
The simultaneous use of Coronal with MAO inhibitors is not recommended due to a significant increase in the hypotensive effect; the break in treatment between taking MAO inhibitors and Coronal should be at least 14 days.
When used simultaneously with Coronal, non-hydrogenated ergot alkaloids and ergotamine increase the risk of developing peripheral circulatory disorders.
When used simultaneously with Coronal, sulfasalazine increases the concentration of bisoprolol in plasma.
When used simultaneously with Coronal, rifampicin shortens the half-life of bisoprolol.
Overdose
Overdose
Symptoms: arrhythmia, ventricular extrasystole, severe bradycardia, AV block, decreased blood pressure, heart failure, cyanosis of fingernails or palms, difficulty breathing, bronchospasm, dizziness, fainting, convulsions.
Treatment: it is necessary to rinse the stomach and prescribe adsorbent drugs. Symptomatic therapy is carried out: in case of developed AV block – intravenous administration of 1-2 mg of atropine, epinephrine (adrenaline) or installation of a temporary pacemaker; for ventricular extrasystole – IV lidocaine (class I A drugs are not used); when blood pressure decreases, the patient should be in the Trendelenburg position; in the absence of symptoms of pulmonary edema – intravenous plasma-substituting solutions, if ineffective – administration of epinephrine (adrenaline), dopamine, dobutamine (to maintain chrono- and inotropic effects and eliminate a pronounced decrease in blood pressure); for heart failure – cardiac glycosides, diuretics, glucagon; for convulsions – intravenous diazepam; for bronchospasm – beta2-adrenergic agonists by inhalation.
Storage conditions
Storage conditions
In a place protected from light, at a temperature of 15–25 °C
Shelf life
Shelf life
2 years
Manufacturer
Manufacturer
Saneka Pharmaceuticals a.s., Slovakia
Additional information
| Shelf life | 2 years |
|---|---|
| Conditions of storage | In a light-protected place at 15-25 °C |
| Manufacturer | Saneka Pharmaceuticals a.s., Slovakia |
| Medication form | pills |
| Brand | Saneka Pharmaceuticals a.s. |
Other forms…
Related products
Buy Coronal, 10 mg 60 pcs with delivery to USA, UK, Europe and over 120 other countries.