No products in the cart.
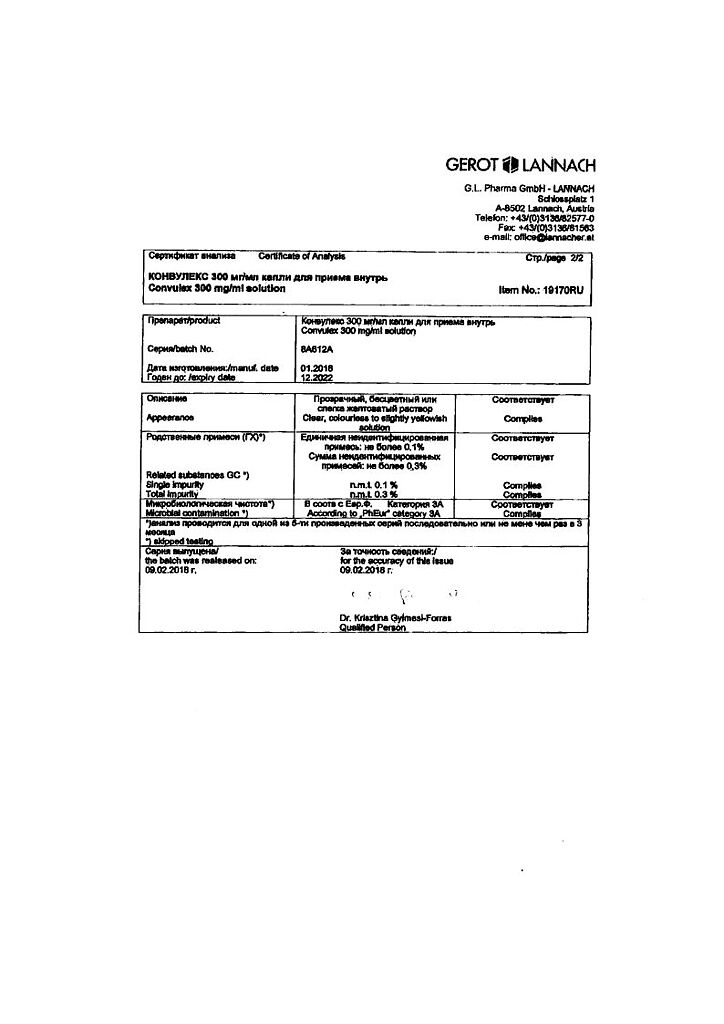
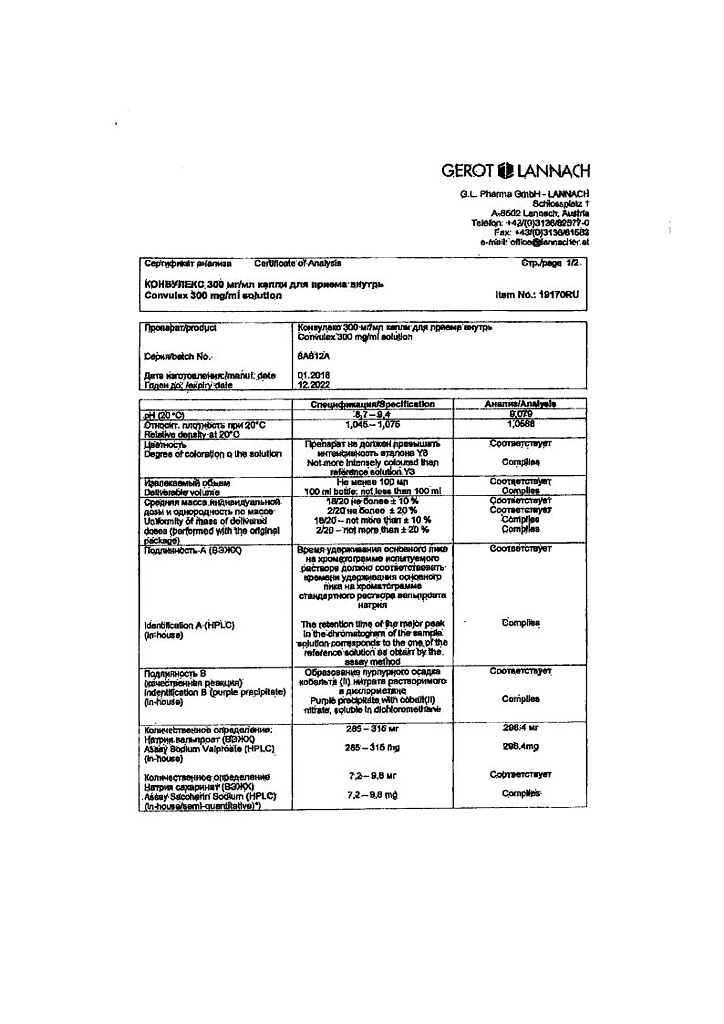
Convullex, drops 300 mg/ml 100 ml
€18.81 €15.67
Description
Convulex® is an antiepileptic drug; it also has central myorelaxant and sedative effects. The mechanism of action is mainly due to inhibition of the enzyme GABA-transferase and increased content of GABA in CNS. GABA prevents pre- and postsynaptic discharges and thereby prevents the spread of seizure activity into the CNS.
In addition, the effects of valproic acid on the GABAA receptors as well as the effects on voltage-dependent sodium channels play a significant role in the drug’s mechanism of action. According to another hypothesis, it acts on post-synaptic receptor sites, mimicking or enhancing the inhibitory effect of GABA. Possible direct effect on membrane activity is due to changes in potassium conductance. Improves the mental state and mood of patients, has antiarrhythmic activity.
Pharmacokinetics
The equilibrium concentration at injection is reached within minutes and can be maintained by slow infusion. Therapeutic plasma concentrations of the drug range from 50-150 mg/L. Valproic acid is bound to plasma proteins by 90-95% at plasma concentrations up to 50 mg/L and by 80-85% at 50-100 mg/L; in uremia, hypoproteinemia and cirrhosis, protein binding is reduced.
Concentration levels in cerebrospinal fluid correlate with the non-protein-bound fraction of the drug, being about 10% of serum levels. Valproic acid penetrates the placental barrier and is excreted with breast milk. The concentration in breast milk is 1-10% of the concentration in maternal plasma.
The drug undergoes glucuronidation and oxidation in the liver; metabolites and unchanged valproic acid (1-3% of dose) are excreted by the kidneys; small amounts are taken out with the feces and exhaled air. T1/2 of the drug is 8 to 20 h in healthy subjects and in monotherapy; when combined with other drugs,
Indications
Indications
Status epilepticus; treatment of epileptic seizures (generalized – absences, myoclonic seizures, tonic-clonic, atonic, mixed; partial – simple, complex, secondary generalized seizures; specific syndromes (West, Lennox-Gastaut).
Pharmacological effect
Pharmacological effect
Konvulex® is an antiepileptic drug that also has a central muscle relaxant and sedative effect. The mechanism of action is primarily due to inhibition of the GABA transferase enzyme and an increase in GABA content in the central nervous system. GABA inhibits pre- and postsynaptic discharges and thereby prevents the spread of seizure activity into the central nervous system.
In addition, in the mechanism of action of the drug, a significant role is played by the effect of valproic acid on GABAA receptors, as well as the effect on voltage-dependent sodium channels. According to another hypothesis, it acts on sites of postsynaptic receptors, simulating or enhancing the inhibitory effect of GABA. A possible direct effect on membrane activity is associated with changes in potassium conductance. Improves the mental state and mood of patients, has antiarrhythmic activity.
Pharmacokinetics
Steady-state concentrations with intravenous administration are achieved within a few minutes and can be maintained by slow infusion. The therapeutic concentration of the drug in blood plasma ranges from 50–150 mg/l. Valproic acid is bound to plasma proteins by 90–95% at plasma concentrations up to 50 mg/l and by 80–85% at concentrations of 50–100 mg/l; with uremia, hypoproteinemia and cirrhosis, protein binding is reduced.
Cerebrospinal fluid concentration levels correlate with the non-protein bound fraction of the drug, being approximately 10% of serum levels. Valproic acid penetrates the placental barrier and is excreted in breast milk. Concentrations in breast milk are 1–10% of maternal plasma concentrations.
The drug undergoes glucuronidation and oxidation in the liver, metabolites and unchanged valproic acid (1–3% of the dose) are excreted by the kidneys, small amounts are excreted in feces and exhaled air. T1/2 of the drug in healthy subjects and with monotherapy is from 8 to 20 hours; when combined with other drugs, T1/2 can be 6–8 hours due to the induction of metabolic enzymes; in patients with impaired liver function and elderly patients – may be significantly longer.
Special instructions
Special instructions
Due to reports of severe and fatal cases of liver failure and pancreatitis when using valproic acid preparations, the following should be kept in mind:
at increased risk are infants and children under 3 years of age with severe epilepsy, often associated with brain damage and congenital metabolic or degenerative diseases;
in most cases, liver dysfunction developed in the first 6 months (usually between 2 and 12 weeks) of treatment, more often with combined antiepileptic treatment;
cases of pancreatitis were observed regardless of the patient’s age and duration of treatment, although the risk of developing pancreatitis decreased with the patient’s age;
insufficiency of liver function with pancreatitis increases the risk of death;
early diagnosis (before the icteric stage) is based mainly on clinical observation – identification of early symptoms such as asthenia, anorexia, extreme fatigue, drowsiness, sometimes accompanied by vomiting and abdominal pain; in this case, a relapse of epileptic seizures may occur against the background of unchanged antiepileptic therapy.
In such cases, you should immediately consult a doctor for a clinical examination and liver function test.
During treatment, especially in the first 6 months, it is necessary to periodically check liver function – the activity of liver transaminases, the level of prothrombin, fibrinogen, coagulation factors, bilirubin concentration, as well as amylase activity (every 3 months, especially when combined with other antiepileptic drugs) and the picture of peripheral blood, in particular, blood platelets.
In patients receiving other antiepileptic drugs, transfer to valproic acid should be carried out gradually, reaching a clinically effective dose after 2 weeks, after which gradual withdrawal of other antiepileptic drugs is possible. In patients not treated with other antiepileptic drugs, a clinically effective dose should be achieved after 1 week.
The risk of side effects from the liver is increased during combination anticonvulsant therapy, as well as in children. Drinks containing ethanol are not allowed.
Before surgery, a general blood test (including platelet count), determination of bleeding time, and coagulogram parameters are required.
If the symptom complex “acute abdomen” occurs during treatment, it is recommended to determine the activity of amylase in the blood before the start of surgery to exclude acute pancreatitis.
During treatment, one should take into account the possible distortion of the results of urine tests in diabetes mellitus (due to an increase in the content of ketone bodies) and indicators of thyroid function.
If any acute serious side effects develop, you should immediately discuss with your doctor the advisability of continuing or stopping treatment.
To reduce the risk of developing dyspeptic disorders, it is possible to take antispasmodics and enveloping agents.
Abruptly stopping taking Convulex may lead to an increase in epileptic seizures.
Impact on the ability to drive vehicles and operate machinery
During the treatment period, care must be taken when driving vehicles and engaging in other potentially hazardous activities that require increased concentration and speed of psychomotor reactions.
Active ingredient
Active ingredient
Valproic acid
Composition
Composition
1 ml
sodium valproate
300 mg
Excipients:
sodium saccharinate – 8.5 mg,
orange flavoring – 2 mg,
hydrochloric acid 37% – q.s. up to pH 9.0,
sodium hydroxide – q.s. up to pH 9.0,
purified water – up to 1 ml.
Pregnancy
Pregnancy
During treatment, pregnancy should be protected.
Experiments on animals revealed the teratogenic effect of valproic acid. The incidence of neural tube defects in children born to women who took valproate in the first trimester of pregnancy is 1-2%. In this regard, it is advisable to use folic acid preparations.
In the first trimester of pregnancy, treatment with Convulex should not be started. If a pregnant woman is already receiving the drug, treatment should not be interrupted due to the risk of increased seizures.
The drug should be used in the lowest effective doses, avoiding combination with other anticonvulsants and, if possible, regularly monitoring the concentration of valproic acid in plasma.
Use in children
Contraindication: children under 3 years of age (for tablets with prolonged action).
Contraindications
Contraindications
hypersensitivity to valproic acid;
severe dysfunction of the liver and/or pancreas;
porphyria;
hemorrhagic diathesis;
severe thrombocytopenia.
With caution:
in children when treated with several antiepileptic drugs;
in children and adolescents with multiple concomitant diseases and severe forms of seizures;
with impaired renal function;
in patients with anamnestic data on diseases of the liver and pancreas;
with suppression of bone marrow hematopoiesis: leukopenia, anemia, thrombocytopenia;
for congenital enzymopathies;
with organic brain lesions;
with hypoproteinemia.
Side Effects
Side Effects
In general, Konvulex® is well tolerated by patients. Side effects are possible mainly when the drug concentration in plasma is above 100 mg/l or during combination therapy.
From the digestive system: nausea, vomiting, gastralgia, decreased or increased appetite, diarrhea, hepatitis, constipation, pancreatitis, up to severe injuries with a fatal outcome (in the first 6 months of treatment, more often for 2-12 weeks).
From the side of the central nervous system: tremor, diplopia, nystagmus, flashing “floaters” before the eyes, changes in behavior, mood or mental state (depression, feeling tired, hallucinations, aggressiveness, hyperactive state, psychosis, unusual agitation, restlessness or irritability), ataxia, dizziness, drowsiness, headache, encephalopathy, dysarthria, enuresis, stupor, impaired consciousness, coma
From the hematopoietic system: anemia, leukopenia, thrombocytopenia, decreased fibrinogen content and platelet aggregation, leading to the development of hypocoagulation (accompanied by prolongation of bleeding time, petechial hemorrhages, bruises, hematomas, bleeding).
From the metabolic side: decrease or increase in body weight.
From the endocrine system: dysmenorrhea, secondary amenorrhea, breast enlargement, galactorrhea.
Laboratory parameters: hypercreatininemia, hyperammonemia, hyperbilirubinemia, slight increase in the activity of liver transaminases, LDH (dose-dependent).
Allergic reactions: skin rash, urticaria, angioedema, photosensitivity, malignant exudative erythema (Stevens-Johnson syndrome).
Other: peripheral edema, hair loss (usually restored after discontinuation of the drug).
Interaction
Interaction
Contraindicated combinations
Mefloquine: risk of epileptic seizures due to increased metabolism of valproic acid and a decrease in its plasma concentration and, on the other hand, the convulsant effect of mefloquine.
St. John’s wort: risk of reducing the concentration of valproic acid in the blood plasma.
Not recommended combinations
Lamotrigine: increased risk of severe skin reactions (toxic epidermal necrolysis). Valproic acid inhibits microsomal liver enzymes that ensure the metabolism of lamotrigine, which slows down its T1/2 to 70 hours in adults and up to 45-55 hours in children and increases plasma concentrations. If the combination is necessary, careful clinical and laboratory monitoring is required.
Combinations requiring special precautions
Carbamazepine: Valproic acid increases the plasma concentration of the active metabolite of carbamazepine to the point of signs of overdose. In addition, carbamazepine enhances the hepatic metabolism of valproic acid and reduces its concentration. These circumstances require the attention of a doctor and determination of drug concentrations in plasma and a possible revision of their doses.
Phenobarbital, primidone: Valproic acid increases plasma concentrations of phenobarbital or primidone to the point of signs of overdose, more often in children. In turn, phenobarbital or primidone enhance the hepatic metabolism of valproic acid and reduce its concentration. Clinical observation is recommended during the first 2 weeks of combined treatment with an immediate reduction in the dose of phenobarbital or primidone if signs of sedation appear, and determination of the level of anticonvulsants in the blood.
Phenytoin: changes in the concentration of phenytoin in plasma are possible; phenytoin increases the hepatic metabolism of valproic acid and reduces its concentration. Clinical observation is recommended, determining the level of anticonvulsants in the blood, changing dosages if necessary.
Clonazepam: The addition of valproic acid to clonazepam in isolated cases may lead to an increase in the severity of absence status.
Ethosuximide: Valproic acid can either increase or decrease the serum concentrations of ethosuximide due to changes in its metabolism. Clinical observation is recommended, determining the level of anticonvulsants in the blood, changing dosages if necessary.
Topiramate: Increases the risk of hyperammonemia and encephalopathy.
Felbamate: increased plasma concentrations of valproic acid by 35-50%, with risk of overdose. Clinical observation, determination of the level of valproic acid in the blood, and changes in the dosage of valproic acid when combined with felbamate and after its discontinuation are recommended.
Neuroleptics, MAO inhibitors, antidepressants, benzodiazepines: neuroleptics, tricyclic antidepressants, MAO inhibitors that lower the seizure threshold reduce the effectiveness of the drug. In turn, valproic acid potentiates the effect of these psychotropic drugs, as well as benzodiazepines.
Cimetidine, erythromycin: suppress the hepatic metabolism of valproic acid and increase its plasma concentration.
Zidovudine: Valproic acid increases the plasma concentration of zidovudine, leading to increased toxicity.
Carbapenems, monobactams: meropenem, panipenem, as well as aztreonam and imipenem reduce the concentration of valproic acid in plasma, which may lead to a decrease in the anticonvulsant effect.
Combinations to consider
Acetylsalicylic acid: increased effects of valproic acid due to its displacement from plasma proteins. Valproic acid enhances the effect of acetylsalicylic acid.
Indirect anticoagulants: valproic acid enhances the effect of indirect anticoagulants; careful monitoring of the prothrombin index is necessary when administered together with vitamin K-dependent anticoagulants.
Nimodipine: increased hypotensive effect of nimodipine due to an increase in its concentration in plasma due to the suppression of its metabolism by valproic acid.
Myelotoxic drugs: increased risk of suppression of bone marrow hematopoiesis.
Ethanol and hepatotoxic drugs: increase the likelihood of developing liver damage.
Other combinations
Oral contraceptives: valproic acid does not induce liver microsomal enzymes and does not reduce the effectiveness of hormonal oral contraceptives.
Overdose
Overdose
Symptoms: nausea, vomiting, dizziness, diarrhea, respiratory dysfunction, muscle hypotonia, hyporeflexia, miosis, coma.
Treatment: gastric lavage (no later than 10-12 hours) followed by the administration of activated charcoal, hemodialysis. Forced diuresis, maintenance of function.
Storage conditions
Storage conditions
In a dry place, protected from light, at a temperature of 15–21 °C
Shelf life
Shelf life
5 years
Manufacturer
Manufacturer
Geroth Pharmaceuticals GmbH, Germany
Additional information
| Shelf life | 5 years |
|---|---|
| Conditions of storage | In a dry, light-protected place at 15-21 °C |
| Manufacturer | Geroth Pharmazoitica GmbH, Germany |
| Medication form | oral drops |
| Brand | Geroth Pharmazoitica GmbH |
Other forms…
Related products
Buy Convullex, drops 300 mg/ml 100 ml with delivery to USA, UK, Europe and over 120 other countries.