No products in the cart.
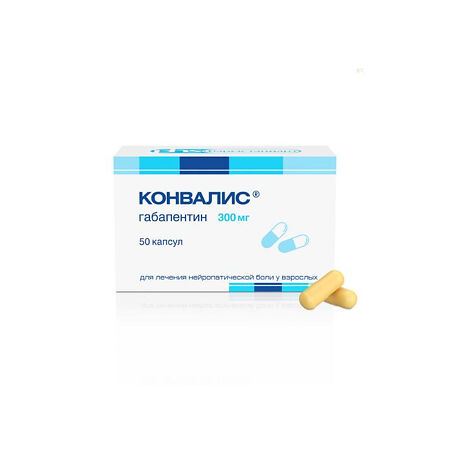
Convalis, 300 mg capsules 50 pcs
€26.76 €20.97
Description
Pharmacotherapeutic group: anti-epileptic drug.
ATX code: N03AX12.
Pharmacological Properties
Pharmacodynamics
The chemical structure of gabapentin is similar to that of the neurotransmitter GABA (gamma-aminobutyric acid), but its mechanism of action differs from other active agents that interact with GABA synapses, such as valproates, barbiturates, benzodiazepines, GABA transaminase inhibitors, GABA reuptake inhibitors, GABA agonists and GABA prodrugs. In in vitro studies with the radioisotope-labeled gabapentin in the rat brain, new regions of binding of the drug to proteins were found, including in the neocortex and hippocampus, which may be relevant to the anticonvulsant and analgesic activity of gabapentin and its derivatives. The binding site of gabapentin was found to be the α-2-δ (alpha-2-delta) subunit of potential-dependent calcium channels.
In clinically relevant concentrations, gabapentin does not bind to other common drug and neurotransmitter receptors present in the brain, including GABAA, GABAB, benzodiazepine, glutamate, glycine and N-methyl-D-aspartate receptors.
Gabapentin under in vitro conditions does not interact with sodium channels, which distinguishes it from phenytoin and carbamazepine. In a number of in vitro test systems the use of gabapentin resulted in a partial reduction in response to the glutamate agonist N-methyl-D-aspartate (NMDA), but only at concentrations greater than 100 μmol/L, which is not achievable in in vivo conditions. Under in vitro conditions, the use of gabapentin results in a slight decrease in monoamine neurotransmitter release.
The exact mechanism of action of gabapentin is unknown.
Pharmacokinetics
absorption
. After oral administration, the maximum plasma concentration of gabapentin is reached within 2-3 hours. Bioavailability of gabapentin tends to decrease with increasing dose of the drug. Absolute bioavailability when taking 300 mg capsules is approximately 60%. Foods, including those with high fat content, have no clinically significant effect on the pharmacokinetic parameters of gabapentin.
The pharmacokinetics of gabapentin do not change with repeated administration of the drug.
Distribution
Gabapentin does not bind to plasma proteins and its volume of distribution is 57.7 L. In patients with epilepsy, the concentration of gabapentin in the cerebrospinal fluid (CSF) is approximately 20% of the minimum equilibrium plasma concentration. Gabapentin is excreted in breast milk.
Biotransformation
There are no data on the metabolism of gabapentin in humans. Gabapentin does not cause induction of nonspecific liver oxidases responsible for drug metabolism.
Elimation
Gabapentin is excreted unchanged exclusively by renal excretion. The half-life of gabapentin is independent of the dose taken and averages 5 to 7 hours.
In elderly patients and patients with impaired renal function the blood plasma clearance of gabapentin is decreased. The elimination constant, plasma clearance and renal clearance of gabapentin are directly proportional to creatinine clearance.
Gabapentin is eliminated from blood plasma by hemodialysis. Patients with impaired renal function or who are on hemodialysis are recommended to adjust the dose of the drug (see section “Dosage and administration”).
Linearity/nonlinearity of pharmacokinetic parameters
The bioavailability of gabapentin decreases with increasing dose taken, resulting in non-linearity of pharmacokinetic parameters that include bioavailability (F), such as Ae%, CL/F, Vd/F. Elimination pharmacokinetics (parameters not including F, such as CLr and T1/2) are better described by a linear model. Equilibrium plasma concentrations of gabapentin are predictable based on single administration kinetics data.
Indications
Indications
Treatment of neuropathic pain in adults aged 18 years and older. Efficacy and safety in patients under 18 years of age have not been established.
Monotherapy for partial seizures with and without secondary generalization in adults and children aged 12 years and older. The effectiveness and safety of monotherapy in children under 12 years of age have not been established.
Pharmacological effect
Pharmacological effect
Pharmacotherapeutic group: antiepileptic drug.
ATX code: N03AX12.
PHARMACOLOGICAL PROPERTIES
Pharmacodynamics
The chemical structure of gabapentin is similar to that of the neurotransmitter GABA (gamma-aminobutyric acid), but its mechanism of action differs from other active substances that interact with GABA synapses, such as valproate, barbiturates, benzodiazepines, GABA transaminase inhibitors, GABA reuptake inhibitors, GABA agonists and GABA prodrugs. In vitro studies with radiolabeled gabapentin in the rat brain revealed new sites of drug binding to proteins, including the neocortex and hippocampus, which may be relevant to the anticonvulsant and analgesic activity of gabapentin and its derivatives. The binding site of gabapentin was found to be the α-2-δ (alpha-2-delta) subunit of voltage-gated calcium channels.
At clinically relevant concentrations, gabapentin does not bind to other common drug and neurotransmitter receptors found in the brain, including GABAA, GABAB, benzodiazepine, glutamate, glycine and N-methyl-D-aspartate receptors.
Gabapentin does not interact with sodium channels in vitro, which distinguishes it from phenytoin and carbamazepine. In a number of in vitro test systems, the use of gabapentin led to a partial decrease in the response to the glutamate agonist N-methyl-D-aspartate (NMDA), but only at concentrations exceeding 100 μmol/L, which is unattainable in in vivo conditions. In vitro, the use of gabapentin leads to a slight decrease in the release of monoamine neurotransmitters.
The exact mechanism of action of gabapentin is unknown.
Pharmacokinetics
Suction
After oral administration, the maximum concentration of gabapentin in the blood plasma is achieved within 2-3 hours. The bioavailability of gabapentin tends to decrease with increasing dosage of the drug. Absolute bioavailability when taking 300 mg capsules is approximately 60%. Food, including food high in fat, does not have a clinically significant effect on the pharmacokinetic parameters of gabapentin.
The pharmacokinetics of gabapentin does not change with repeated administration of the drug.
Distribution
Gabapentin does not bind to plasma proteins and its volume of distribution is 57.7 L. In patients with epilepsy, the concentration of gabapentin in the cerebrospinal fluid (CSF) is approximately 20% of the minimum steady-state plasma concentration. Gabapentin is excreted into breast milk.
Biotransformation
There are no data on the metabolism of gabapentin in humans. Gabapentin does not induce nonspecific liver oxidases responsible for drug metabolism.
Removal
Gabapentin is excreted unchanged exclusively by renal excretion. The half-life of gabapentin does not depend on the dose taken and averages from 5 to 7 hours.
In elderly people and patients with impaired renal function, the clearance of gabapentin from blood plasma is reduced. The elimination constant, plasma clearance and renal clearance of gabapentin are directly proportional to creatinine clearance.
Gabapentin is removed from blood plasma during hemodialysis. For patients with impaired renal function or those on hemodialysis, a dose adjustment of the drug is recommended (see section “Method of administration and dosage”).
Linearity/nonlinearity of pharmacokinetic parameters
The bioavailability of gabapentin decreases with increasing dose, which entails non-linearity of pharmacokinetic parameters, which include the bioavailability index (F) in the calculation, for example, Ae%, CL/F, Vd/F. Elimination pharmacokinetics (non-F parameters such as CLr and T1/2) are better described by a linear model. Steady-state plasma concentrations of gabapentin are predictable based on single-dose kinetic data.
Special instructions
Special instructions
Antiepileptic drugs, including gabapentin, may increase the risk of suicidal thoughts or behavior. A meta-analysis of randomized placebo-controlled trials of antiepileptic drugs demonstrated a small increase in the risk of suicidal ideation and behavior. The mechanism for increasing the risk of developing suicidal ideation is unknown, but for gabapentin it cannot be excluded.
Therefore, patients receiving these drugs should be closely monitored for new or worsening depression, the emergence of suicidal thoughts or behavior, and any changes in behavior. If signs of suicidal thoughts or behavior occur, patients or their caregivers should seek medical attention.
Acute pancreatitis
If acute pancreatitis develops while taking gabapentin, the possibility of discontinuing the drug should be assessed.
Convulsions (withdrawal syndrome)
Although withdrawal syndrome accompanied by the development of seizures has not been observed during treatment with gabapentin, abrupt cessation of anticonvulsant drug therapy in patients with epilepsy may provoke the development of status epilepticus.
As with other antiepileptic drugs, gabapentin may cause an increase in the frequency of seizures or the appearance of a different type of seizure.
As with other anticonvulsants, attempts to discontinue all concomitant antiepileptic drugs in order to initiate gabapentin monotherapy in cases of treatment refractory in patients taking multiple anticonvulsants are generally unsuccessful.
Gabapentin is not thought to be effective for primary generalized seizures, such as absence seizures, and may even worsen such seizures in some patients. In this regard, gabapentin should be used with caution in patients with mixed seizures, including absence seizures.
Elderly patients
Systematic studies have not been conducted in patients aged 65 years and older taking gabapentin. In a double-blind study of gabapentin for neuropathic pain, patients aged 65 years and older had a higher incidence of somnolence, peripheral edema, and asthenia compared with patients aged <65 years. Apart from these results, clinical examination of this group of patients showed that their side effect profile did not differ from the rest.
Children
The effect of long-term therapy (more than 36 weeks) with gabapentin on the learning ability, intelligence and development of the child has not been sufficiently studied. The ratio of possible risks and benefits should be assessed when prescribing long-term therapy.
Abuse and addiction
The post-marketing surveillance database contains reports of cases of drug abuse and dependence. As with any drug that affects the central nervous system, clinicians should carefully review patients’ drug abuse history and monitor patients for possible signs of gabapentin abuse (eg, drug diversion, development of resistance to gabapentin therapy, inappropriate dosage increases).
DRESS syndrome
Severe life-threatening hypersensitivity reactions, such as drug rash with associated eosinophilia and systemic symptoms, have been reported while taking antiepileptic drugs, including gabapentin. It must be remembered that early signs of a hypersensitivity reaction, such as fever, lymphadenopathy, can develop even in the absence of a skin rash. If such symptoms occur, immediate examination of the patient is necessary. If no other reason is found other than the use of gabapentin, the use of the drug should be discontinued.
Anaphylaxis
Taking gabapentin can lead to the development of anaphylaxis. The following symptoms and signs were noted in cases of anaphylaxis while taking gabapentin – difficulty breathing, swelling of the lips, throat and tongue, and a marked decrease in blood pressure was also noted, requiring urgent medical intervention. Patients should be warned that if signs or symptoms of anaphylaxis develop, they should stop taking the drug and seek medical attention.
Laboratory tests
False-positive results have been reported when gabapentin and other anticonvulsants were used concomitantly with Ames N-Multistix SG® urinary protein test strips. To determine protein in urine, it is recommended to use the more specific method of precipitation with sulfosalicylic acid.
Effect on the central nervous system
During treatment with gabapentin, cases of dizziness and drowsiness have been observed, which may increase the likelihood of accidental injury (from a fall). Cases of confusion, loss of consciousness and mental impairment have also been reported during the post-marketing period. Therefore, patients should be advised to use caution until they are aware of the possible effects of this drug.
When used simultaneously with opioid analgesics, an increase in the concentration of gabapentin in the blood plasma may be observed. Therefore, the patient needs to be closely monitored for the development of signs of CNS depression, such as drowsiness, sedation, and respiratory depression. Doses of gabapentin or opioid analgesics should be reduced.
Combined use with antacids
It is recommended to take gabapentin approximately 2 hours after taking the antacid.
INFLUENCE ON THE ABILITY TO DRIVE VEHICLES AND MECHANISMS
While taking the drug, patients are not recommended to drive vehicles or use potentially dangerous equipment until it is confirmed that the drug does not have a negative effect on the performance of these functions.
Gabapentin affects the central nervous system and may cause dizziness, drowsiness, confusion, loss of consciousness, or other central nervous system symptoms. Even if mild or moderate, these undesirable effects may pose a hazard to patients driving vehicles or operating machinery. This likelihood is especially high at the beginning of treatment or after increasing the dose of gabapentin.
Active ingredient
Active ingredient
Gabapentin
Composition
Composition
active substance: gabapentin – 300.0 mg;
excipients: lactose monohydrate – 66.0 mg, pregelatinized corn starch – 30.0 mg, talc – 3.0 mg, magnesium stearate – 1.0 mg.
The mass of the capsule contents is 400.0 mg.
Composition of the capsule shell
Hard gelatin capsules No. 0 – 96.0 mg.
Body and cap: titanium dioxide (E 171) – 2.0000%, yellow iron oxide dye (E 172) – 0.6286%, gelatin – up to 100%.
The total weight of the capsule with contents is 496.0 mg.
Pregnancy
Pregnancy
General risk due to epilepsy and antiepileptic drugs
The risk of having children with congenital anomalies in mothers who are treated with anticonvulsants increases 2-3 times. Most often, cleft lip and palate, malformations of the cardiovascular system and neural tube defects are observed. However, taking multiple anticonvulsants may be associated with a greater risk of developing defects than in the case of monotherapy. Therefore, if possible, one of the anticonvulsants should be used. Women of childbearing age, and all women who may become pregnant, should seek advice from a qualified healthcare professional. If a woman is planning a pregnancy, the need to continue anticonvulsant therapy should be re-evaluated. However, anticonvulsant drugs should not be discontinued abruptly, as this can lead to resumption of seizures with serious consequences for the mother and child. In rare cases, developmental delays have been observed in children whose mothers have epilepsy. However, it is impossible to determine whether developmental delay is due to genetic or social factors, maternal illness or anticonvulsant therapy.
Risk associated with gabapentin
There are no data on the use of the drug in pregnant women. Experiments on animals showed the toxicity of the drug to the fetus. People have no data regarding the possible risk. Therefore, gabapentin should be used during pregnancy only if the expected benefit to the mother justifies the possible risk to the fetus.
In the cases that have been reported, it is impossible to say with certainty whether or not the use of gabapentin during pregnancy is accompanied by an increased risk of malformations, firstly, due to the presence of epilepsy itself, and secondly, due to the use of other anticonvulsants.
Breast-feeding
Gabapentin is excreted in breast milk and its effect on the nursing baby is unknown, so Convalis® should be prescribed during breastfeeding only if the benefit to the mother clearly outweighs the risk to the infant.
In animal studies, there was no effect of gabapentin on fertility.
Contraindications
Contraindications
Hypersensitivity to gabapentin or auxiliary components of the drug.
Epilepsy: use as monotherapy for partial seizures with and without secondary generalization in children under 12 years of age.
Neuropathic pain: for the treatment of neuropathic pain in children and adolescents under 18 years of age.
Lactase deficiency, lactose intolerance, glucose-galactose malabsorption.
WITH CAUTION
Renal failure (see “Dosage and Administration”).
Side Effects
Side Effects
Adverse effects observed in clinical studies of patients with epilepsy (using gabapentin as monotherapy or in combination with other anticonvulsants) or neuropathic pain are presented below and categorized by organ system and frequency.
The frequency category was defined as follows: very often (≥1/10); often (from ≥1/100 to <1/10); uncommon (from ≥1/1000 to <1/100); rare (from ≥1/10000 to <1/1000); very rare (<1/10,000). If the frequency category was different between studies, the side effect was assigned a higher category.
Side effects reported during the use of the drug after registration were assigned a frequency category of “unknown” (frequency cannot be calculated based on available data).
In each frequency section, side effects are presented in order of decreasing severity.
Infectious and parasitic diseases: very often – viral infections; often – pneumonia, respiratory tract infection, urinary tract infection, other types of infection, otitis media.
Disorders of the blood and lymphatic system: often – leukopenia; unknown – thrombocytopenia.
Immune system disorders: uncommon – allergic reactions, including urticaria; unknown – hypersensitivity, including systemic reactions such as fever, rash, hepatitis, lymphadenopathy, eosinophilia and others.
Metabolic and nutritional disorders: often – anorexia, increased appetite.
Mental disorders: often – hostility, confusion, depression, anxiety, nervousness, impaired thinking, emotional lability; infrequently – deterioration of mental state; unknown – hallucinations.
Nervous system disorders: very often – drowsiness, dizziness, ataxia; often – convulsions, hyperkinesia, dysarthria, amnesia, tremor, insomnia, headache, sensory disturbances (for example, paresthesia, hypesthesia), loss of coordination, nystagmus, strengthening, weakening or absence of reflexes; infrequently – hypokinesia; rarely – loss of consciousness; unknown – other movement disorders (for example, choreoathetosis, dyskinesia and dystonia).
Visual disorders: often – visual impairment (such as amblyopia, diplopia).
Hearing and labyrinthine disorders: often – vertigo; unknown – tinnitus.
Cardiac disorders: uncommon – palpitations.
Vascular disorders: often – symptoms of vasodilation or arterial hypertension.
Disorders of the respiratory system, chest and mediastinal organs: often – shortness of breath, bronchitis, pharyngitis, cough, rhinitis.
Gastrointestinal disorders: often – constipation, diarrhea, dry mucous membrane of the mouth or pharynx, dyspepsia, flatulence, nausea, vomiting, abdominal pain, dental disease, gingivitis; unknown – pancreatitis.
Liver and biliary tract disorders: unknown – hepatitis, jaundice.
Disorders of the skin and subcutaneous tissues: often – swelling of the face, purpura (most often it was described as bruising resulting from physical trauma), skin rash, acne, itching of the skin; unknown – Stevens-Johnson syndrome, angioedema, erythema multiforme, alopecia, drug skin rash, including eosinophilia and systemic reactions (see section “Special instructions”).
Musculoskeletal and connective tissue disorders: often – myalgia, arthralgia, back pain, muscle twitching; unknown – rhabdomyolysis, myoclonus.
Renal and urinary tract disorders: unknown – urinary incontinence, acute renal failure.
Disorders of the genital organs and mammary gland: often – impotence; unknown – increase in the volume of the mammary glands, gynecomastia, sexual dysfunction (including changes in libido, ejaculation disorders and anorgasmia).
General disorders and disorders at the injection site: very often – fatigue, fever; often – peripheral edema, gait disturbance, asthenia, pain of various localizations, general malaise, flu-like syndrome; uncommon – generalized edema; unknown – withdrawal syndrome (the most frequently reported side effects were anxiety, insomnia, nausea, pain of various localizations and increased sweating), chest pain. There have been cases of sudden unexplained death, the connection of which with gabapentin treatment has not been established.
Laboratory and instrumental data: often – decreased concentration of white blood cells, increased body weight; infrequently – increased activity of alanine aminotransferase, aspartate aminotransferase and bilirubin concentration in the blood plasma, hyperglycemia; rarely – hypoglycemia (mainly in patients with diabetes mellitus); unknown – hyponatremia, increased creatine phosphokinase activity.
Injuries, intoxications and complications of manipulations: often – injuries, fractures, abrasions associated with falls.
There are reports of the development of acute pancreatitis during gabapentin therapy. The causal relationship with gabapentin remains unclear (see section “Special instructions”).
There are reports of cases of myopathy with increased creatine kinase activity in patients with end-stage renal failure undergoing hemodialysis.
Cases of respiratory tract infection, otitis media, bronchitis and seizures were reported only in clinical studies. In addition, clinical studies have reported cases of aggressive behavior and hyperkinesis in children.
If any of the side effects indicated in the instructions get worse, or you notice any other side effects not listed in the instructions, tell your doctor.
Interaction
Interaction
There have been spontaneous and literature reports of possible respiratory depression and/or symptoms of sedation associated with gabapentin and opioid analgesics. In some of these cases, the authors attributed these symptoms to the concomitant use of gabapentin and opioids, especially in elderly patients.
When gabapentin 600 mg was administered 2 hours after administration of morphine 60 mg extended-release capsules, there was a 44% increase in mean gabapentin AUC compared to gabapentin monotherapy, which was associated with an increase in pain threshold (cold pressor test). The clinical significance of this change has not been established; the pharmacokinetic characteristics of morphine did not change. The side effects of morphine when taken together with gabapentin did not differ from those when morphine was taken together with placebo. The extent to which these drugs interact at other doses is unknown.
No interactions were observed between gabapentin and phenobarbital, phenytoin, valproic acid and carbamazepine. The pharmacokinetics of gabapentin at steady state are similar in healthy subjects and patients receiving other anticonvulsants.
The simultaneous use of gabapentin with oral contraceptives containing norethisterone and/or ethinyl estradiol is not accompanied by changes in the pharmacokinetics of both components.
The simultaneous use of gabapentin with antacids containing aluminum and magnesium is accompanied by a decrease in the bioavailability of gabapentin by approximately 24% (see section “Special Instructions”).
Probenecid does not affect the renal excretion of gabapentin.
The small decrease (14%) in renal excretion of gabapentin during concomitant use of cimetidine is probably not clinically significant.
With simultaneous use of naproxen (250 mg) and gabapentin (125 mg), an increase in gabapentin absorption from 12% to 15% was observed. Gabapentin has no effect on the pharmacokinetic parameters of naproxen. The indicated doses of drugs are less than the minimum therapeutic ones. The simultaneous use of these drugs in large doses has not been studied.
Overdose
Overdose
With a single dose of 49 g of gabapentin, the following symptoms were observed: dizziness, double vision, speech impairment, drowsiness, loss of consciousness, lethargy and mild diarrhea, which completely disappeared with symptomatic therapy.
It should be taken into account that after taking high doses of gabapentin, its absorption in the intestine decreases.
An overdose of gabapentin may result in coma, especially with the simultaneous use of other drugs that depress the central nervous system.
Although gabapentin can be eliminated by hemodialysis, current experience shows that this is not usually necessary. Hemodialysis may be indicated for patients with severe renal impairment.
Storage conditions
Storage conditions
In a dry place, protected from light, at a temperature not exceeding 25 °C.
Keep out of the reach of children.
Shelf life
Shelf life
3 years. Do not use after the expiration date stated on the package.
Manufacturer
Manufacturer
Pharmstandard-Leksredstva, Russia
Additional information
| Shelf life | 3 years. Do not use after the expiration date stated on the package. |
|---|---|
| Conditions of storage | Store in a dry, light-protected place at a temperature not exceeding 25 °С. Keep out of reach of children. |
| Manufacturer | Pharmstandard-Leksredstva, Russia |
| Medication form | capsules |
| Brand | Pharmstandard-Leksredstva |
Related products
Buy Convalis, 300 mg capsules 50 pcs with delivery to USA, UK, Europe and over 120 other countries.