No products in the cart.
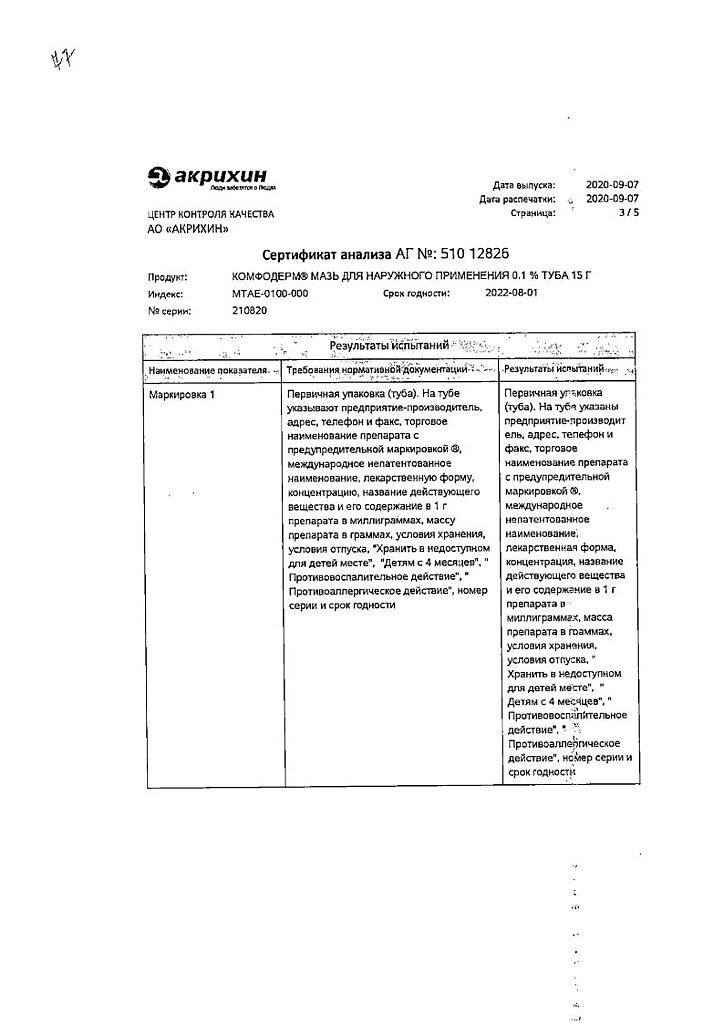
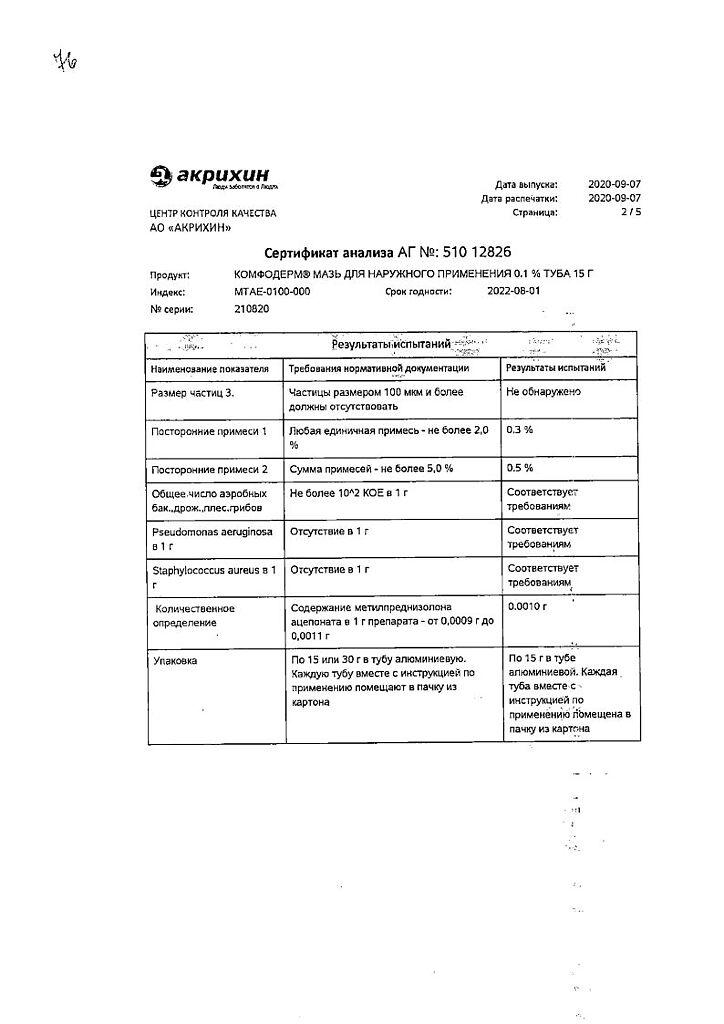
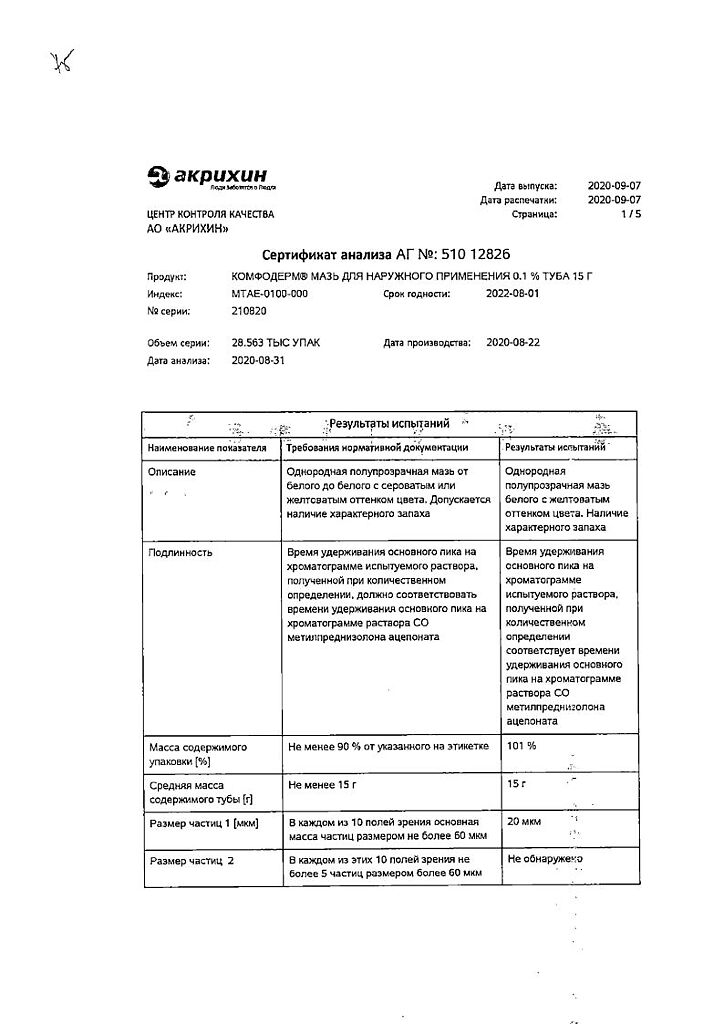
Comfoderm ointment 0.1% 15 g
€12.62 €10.52
Description
The active ingredient of Comfoderm® – methylprednisolone aceponate – is a nonhalogenated steroid.
Pharmacodynamics
In external use methylprednisolone aceponate suppresses inflammatory and allergic skin reactions as well as reactions associated with increased proliferation, resulting in reduction of objective symptoms of inflammation (erythema, edema, mottling, etc.etc.) and subjective sensations (itching, irritation, pain, etc.).
When methylprednisolone aceponate is applied topically at the recommended dose, systemic effects are minimal in both humans and animals. After repeated application of the drug to large surfaces (40-60% of the skin surface), as well as after application under the occlusive dressing no adrenal disturbances are observed: the plasma cortisol level and its circadian rhythm remain within normal limits, there is no reduction of cortisol level in daily urine.
Methylprednisolone aceponate (especially its main metabolite, 6α-methylprednisolone-17-propionate) binds to intracellular glucocorticosteroid receptors. The steroid-receptor complex binds to specific DNA sites of immune response cells, thus causing a series of biological effects. In particular, binding of the steroid-receptor complex to the DNA of the immune response cells leads to induction of macrocortin synthesis.
Macrocortin inhibits the release of arachidonic acid and thus the formation of inflammatory mediators such as prostaglandins and leukotrienes. Inhibition by glucocorticosteroids of the synthesis of vasodilatory prostaglandites and potentiation of the vasoconstrictor effect of adrenaline, lead to a vasoconstrictor effect
Pharmacokinetics
Methylprednisolone aceponate is hydrolyzed in the epidermis and dermis. The main and most active metabolite is 6α-methylprednisolone-17-propionate, which has a significantly higher affinity for glucocorticosteroid receptors in the skin, indicating the presence of its “bioactivation” in the skin.
The extent of percutaneous absorption depends on the condition of the skin, the dosage form and the method of administration (with or without an occlusive dressing).
After entering the systemic bloodstream, 6α-methylprednisolone-17-propionate rapidly conjugates with glucuronic acid and is thus inactivated in the form of 6α-methylprednisolone-17-propionate glucuronide. Metabolites of methylprednisolone aceponate are eliminated mainly by the kidneys with a half-life of about 16 hours. Methylprednisolone aceponate and its metabolites do not cumulate in the body.
Indications
Indications
Inflammatory skin diseases sensitive to therapy with topical glucocorticosteroids:
atopic dermatitis, neurodermatitis, childhood eczema;
true eczema;
microbial eczema;
occupational eczema;
simple contact dermatitis;
allergic (contact) dermatitis;
dyshidrotic eczema.
Pharmacological effect
Pharmacological effect
The active component of Comfoderm®, methylprednisolone aceponate, is a non-halogenated steroid.
Pharmacodynamics
When applied externally, methylprednisolone aceponate suppresses inflammatory and allergic skin reactions, as well as reactions associated with increased proliferation, which leads to a reduction in objective symptoms of inflammation (erythema, swelling, weeping, etc.) and subjective sensations (itching, irritation, pain, etc.).
When methylprednisolone aceponate is used topically at the recommended dose, the systemic effect is minimal in both humans and animals. After repeated application of the drug to large surfaces (40-60% of the skin surface), as well as application under an occlusive dressing, no dysfunction of the adrenal glands is observed: the level of cortisol in plasma and its circadian rhythm remain within normal limits, and there is no decrease in the level of cortisol in daily urine.
Methylprednisolone aceponate (especially its main metabolite, 6α-methylprednisolone-17-propionate) binds to intracellular glucocorticosteroid receptors. The steroid receptor complex binds to specific regions of the DNA of immune response cells, thereby causing a series of biological effects. In particular, the binding of the steroid receptor complex to DNA by immune response cells leads to the induction of macrocortin synthesis.
Macrocortin inhibits the release of arachidonic acid and, thereby, the formation of inflammatory mediators such as prostaglandins and leukotrienes. Inhibition of the synthesis of vasodilating prostaglandites by glucocorticosteroids and potentiation of the vasoconstrictor effect of adrenaline lead to a vasoconstrictor effect
Pharmacokinetics
Methylprednisolone aceponate is hydrolyzed in the epidermis and dermis. The main and most active metabolite is 6α-methylprednisolone-17-propionate, which has a significantly higher affinity for glucocorticosteroid receptors of the skin, which indicates the presence of its “bioactivation” in the skin.
The degree of percutaneous absorption depends on the condition of the skin, the dosage form and the route of administration (with or without an occlusive dressing).
After entering the systemic circulation, 6α-methylprednisolone-17-propionate quickly conjugates with glucuronic acid and is thus inactivated in the form of 6α-methylprednisolone-17-propionate glucuronide. Metabolites of methylprednisolone aceponate are eliminated mainly by the kidneys with a half-life of about 16 hours. Methylprednisolone aceponate and its metabolites do not accumulate in the body
Special instructions
Special instructions
In the presence of bacterial complications and/or dermatomycosis, in addition to therapy with Comfoderm®, it is necessary to carry out specific antibacterial and/or antimycotic treatment. Avoid contact of the drug with the eyes. As with the use of systemic glucocorticosteroids, glaucoma may develop after topical use of glucocorticosteroids (for example, when using large doses or very long-term use of occlusive dressings or application to the skin around the eyes).
Active ingredient
Active ingredient
Methylprednisolone aceponate
Composition
Composition
100 g of ointment contains:
Active substance:
methylprednisolone aceponate in terms of 100% substance – 0.1 g;
Excipients:
Vaseline – 44.7 g;
liquid paraffin – 34.1 g;
castor bean seed oil – 3.2 g;
white beeswax – 17.9 g.
Contraindications
Contraindications
Tuberculous or syphilitic processes in the area of application of the drug;
viral diseases (for example, chickenpox, herpes zoster) in the area where the drug is applied;
rosacea, perioral dermatitis in the area of application of the drug;
children up to 4 months;
areas of skin with manifestations of a reaction to vaccination;
hypersensitivity to the components of the drug.
Side Effects
Side Effects
The incidence of side effects is classified according to the recommendations of the World Health Organization (WHO): very common (≥ 10%), common (≥ 1%, < 10%), uncommon (≥ 0.1%, < 1%), rare (≥ 0.01%, < 0.1%), very rare (< 0.01%), frequency unknown (it is not possible to estimate the frequency of occurrence).Disorders of the skin and subcutaneous tissues: rarely – perioral dermatitis, skin depigmentation, allergic reactions to the components of the drug; frequency unknown – skin atrophy, telangiectasia, striae, acne-like skin changes (when using the drug for more than 4 weeks and/or on an area of 10% or more of the body surface).General disorders and disorders at the injection site: rarely – folliculitis, hypertrichosis; very rarely – itching, burning, erythema, formation of a vesicular rash; frequency unknown – systemic effects due to the absorption of glucocorticosteroids (when using the drug for more than 4 weeks and/or on an area of 10% or more of the body surface). If any of the side effects indicated in the instructions worsen, or any other side effects not specified in the instructions are noted, you should immediately inform your doctor.
Interaction
Interaction
Not studied.
Overdose
Overdose
When studying the acute toxicity of methylprednisolone aceponate, no risk of acute intoxication was identified with excessive single dermal application (applying the drug over a large area under conditions favorable for absorption) or unintentional ingestion. With excessively long and/or intensive use of glucocorticosteroids for external use, skin atrophy (thinning of the skin, telangiectasia, striae) may develop.
If atrophy occurs, the drug must be discontinued.
Storage conditions
Storage conditions
At a temperature not higher than 25°C.
Keep out of the reach of children.
Shelf life
Shelf life
2 years
Manufacturer
Manufacturer
Akrikhin JSC, Russia
Additional information
| Shelf life | 2 years |
|---|---|
| Conditions of storage | At a temperature not exceeding 25°C. Keep out of reach of children. |
| Manufacturer | Akrihin HFC JSC, Russia |
| Medication form | topical ointment |
| Brand | Akrihin HFC JSC |
Other forms…
Related products
Buy Comfoderm ointment 0.1% 15 g with delivery to USA, UK, Europe and over 120 other countries.