No products in the cart.
Clotrimazole, vaginal cream 2% 20 g
€12.79 €10.66
Description
Pharmacotherapeutic group:
antifungal agent.
ATX code: G01AF02
Pharmacological properties
Pharmacodynamics
Clotrimazole is a broad spectrum antifungal agent for local use. Antimycotic effect of the active active substance clotrimazole (imidazole derivative) is associated with disruption of ergosterol synthesis, a component of the cell membrane of fungi, which changes membrane permeability and causes subsequent cell lysis.
In low concentrations it is fungistatic and in high concentrations it is fungicidal, and not only on proliferating cells. In fungicidal concentrations, it interacts with mitochondrial and peroxidase enzymes, resulting in an increase in hydrogen peroxide concentration to toxic levels, which also helps destroy fungal cells.
It is effective against dermatophytes, yeasts, molds and protozoa. It has antimicrobial action against Gram-positive (Streptococcus spp., Staphylococcus spp.) and anaerobes (Bacteroides spp., Gardnerella vaginalis).
Clotrimazole has no effect on lactobacilli. In vitro at a concentration of 0.5-10 µg/ml clotrimazole inhibits the reproduction of bacteria of the family Corinebacteria and Gram-positive cocci (except enterococci); it has a trichomonacid effect at a concentration of 100 µg/ml.
Pharmacokinetics
When using clotrimazole intravaginally, absorption is 3 to 10% of the administered dose. High concentrations in vaginal secretion and low concentrations in blood persist for 48 to 72 hours. It is metabolized in the liver to inactive metabolites, which are excreted by the kidneys and through the intestine.
Indications
Indications
Genital infections caused by yeast-like fungi of the genus Candida (vulvovaginal candidiasis).
If necessary, please consult
with your doctor before using the medicine.
Pharmacological effect
Pharmacological effect
Pharmacotherapeutic group:
antifungal agent.
ATX code: G01AF02
Pharmacological properties
Pharmacodynamics
Clotrimazole is a broad-spectrum antifungal agent for topical use. The antimycotic effect of the active ingredient clotrimazole (imidazole derivative) is associated with a disruption in the synthesis of ergosterol, which is part of the cell membrane of fungi, which changes the permeability of the membrane and causes subsequent cell lysis.
In small concentrations it has a fungistatic effect, and in large concentrations it has a fungicidal effect, and not only on proliferating cells. At fungicidal concentrations, it interacts with mitochondrial and peroxidase enzymes, resulting in an increase in the concentration of hydrogen peroxide to a toxic level, which also contributes to the destruction of fungal cells.
Effective against dermatophytes, yeast-like fungi, molds and protozoa. It has an antimicrobial effect against gram-positive (Streptococcus spp., Staphylococcus spp.) and anaerobes (Bacteroides spp., Gardnerella vaginalis).
Clotrimazole has no effect on lactobacilli. In vitro, at a concentration of 0.5-10 μg/ml, clotrimazole inhibits the proliferation of bacteria of the Corinebacteria family and gram-positive cocci (with the exception of enterococci); has a trichomonacid effect at a concentration of 100 μg/ml.
Pharmacokinetics
When using clotrimazole intravaginally, absorption is 3–10% of the administered dose. High concentrations in vaginal secretions and low concentrations in the blood persist for 48–72 hours. Metabolized in the liver to inactive metabolites, excreted from the body by the kidneys and through the intestines.
Special instructions
Special instructions
Read the instructions for use carefully before you start using the drug. Save the instructions, you may need them again. If you have any questions, consult your doctor.
The medicine you are using is intended for you personally and should not be given to others as it may cause harm to them even if they have the same symptoms as you.
If allergic reactions or irritation occur at the injection site, treatment is stopped.
The drug should not be used during menstruation; it is advisable to start treatment after menstruation.
In case of simultaneous infection of the labia and adjacent areas (candidal vulvitis), additional local treatment with clotrimazole should be carried out.
During pregnancy, treatment with vaginal cream is carried out without an applicator.
To prevent infection, simultaneous treatment of sexual partners is necessary.
During the treatment period, it is recommended to abstain from sexual intercourse.
During treatment, it is recommended to use contraception. The risk of condom or diaphragm rupture increases when used simultaneously with the drug. It is necessary to use reliable methods of contraception.
To prevent reinfection, hygiene rules should be observed.
If clinical signs of infection persist after completion of treatment, repeat microbiological testing should be performed to confirm the diagnosis.
Impact on the ability to drive vehicles and machinery
The drug does not affect the ability to drive vehicles or engage in other potentially hazardous activities that require increased concentration and speed of psychomotor reactions.
Active ingredient
Active ingredient
Clotrimazole
Composition
Composition
100 g of cream contains:
Active substance:
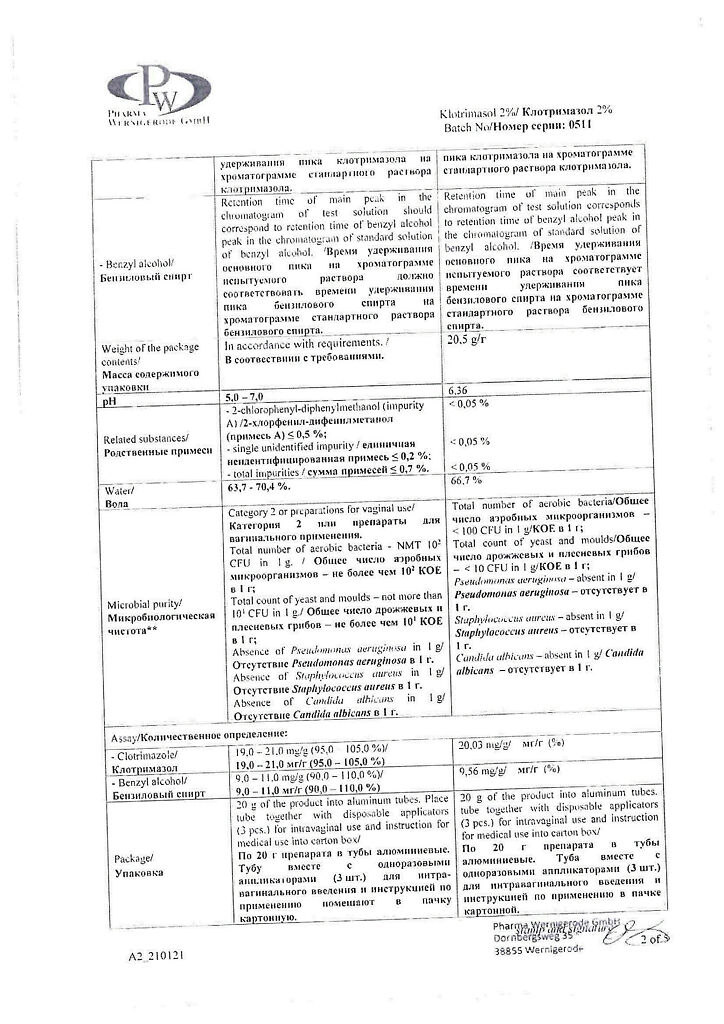
clotrimazole – 2.0 g;
Excipients:
sorbitan stearate – 2.0 g,
cetyl palmitate – 3.0 g,
cetostearyl alcohol [cetyl alcohol 60% and stearyl alcohol 40%] – 10.0 g,
polysorbate-60 – 1.5 g,
octyldodecanol – 13.5 g,
benzyl alcohol – 1.0 g,
purified water – 67.0 g.
Pregnancy
Pregnancy
Use in the first trimester of pregnancy is contraindicated. The question of the advisability of using the drug in the II-III trimesters of pregnancy should be decided individually after consulting a doctor.
The use of the drug during breastfeeding is allowed only in
if, in the opinion of the physician, there is potential benefit from the use of the cream for
mother exceeds the possible risk to the child.
Contraindications
Contraindications
Hypersensitivity to clotrimazole or other components of the drug, especially cetostearyl alcohol;
menstruation period;
I trimester of pregnancy.
With caution
Breastfeeding period.
Side Effects
Side Effects
According to the World Health Organization (WHO), adverse effects are classified according to their frequency as follows: very common (≥ 1/10), common (≥ 1/100, ˂1/10), uncommon (≥ 1/1000, ˂1/100), rare (≥ 1/10000, ˂1/1000) and very rare (˂ 1/10000); frequency unknown (the frequency of events cannot be determined from the available data).
Immune system disorders:
frequency unknown: allergic reactions with symptoms such as urticaria, fainting, hypotension, shortness of breath.
Gastrointestinal disorders:
frequency unknown: abdominal pain.
Disorders of the skin and subcutaneous tissues:
frequency unknown: in case of hypersensitivity to the active substance or another component of the drug, such as cetostearyl alcohol, allergic reactions may occur.
Renal and urinary tract disorders:
frequency unknown: frequent urination, intercurrent cystitis.
Genital disorders:
frequency unknown: itching, burning, hyperemia and swelling of the vaginal mucosa, ulceration of the vaginal mucosa, rash, pain in the pelvic area.
Others:
frequency unknown: headache, gastralgia.
If any of the side effects indicated in the instructions get worse or you notice any other side effects not listed in the instructions, tell your doctor.
Interaction
Interaction
When administered vaginally, clotrimazole reduces the activity of amphotericin B and other polyene antibiotics.
When used simultaneously with natamycin and nystatin, the activity of clotrimazole may be reduced.
Concomitant use of intravaginal clotrimazole and tacrolimus,
sirolimus orally may lead to increased concentrations of the latter in
blood plasma, therefore patients should be monitored for the development
symptoms of their overdose, if necessary, measuring concentrations in
blood plasma.
Overdose
Overdose
In case of overdose, the following symptoms are possible: dizziness, nausea, vomiting.
Treatment. There is no specific antidote. In case of accidental intake
Symptomatic treatment should be carried out internally.
Storage conditions
Storage conditions
At a temperature not exceeding 25 °C.
Keep out of the reach of children.
Shelf life
Shelf life
3 years.
Manufacturer
Manufacturer
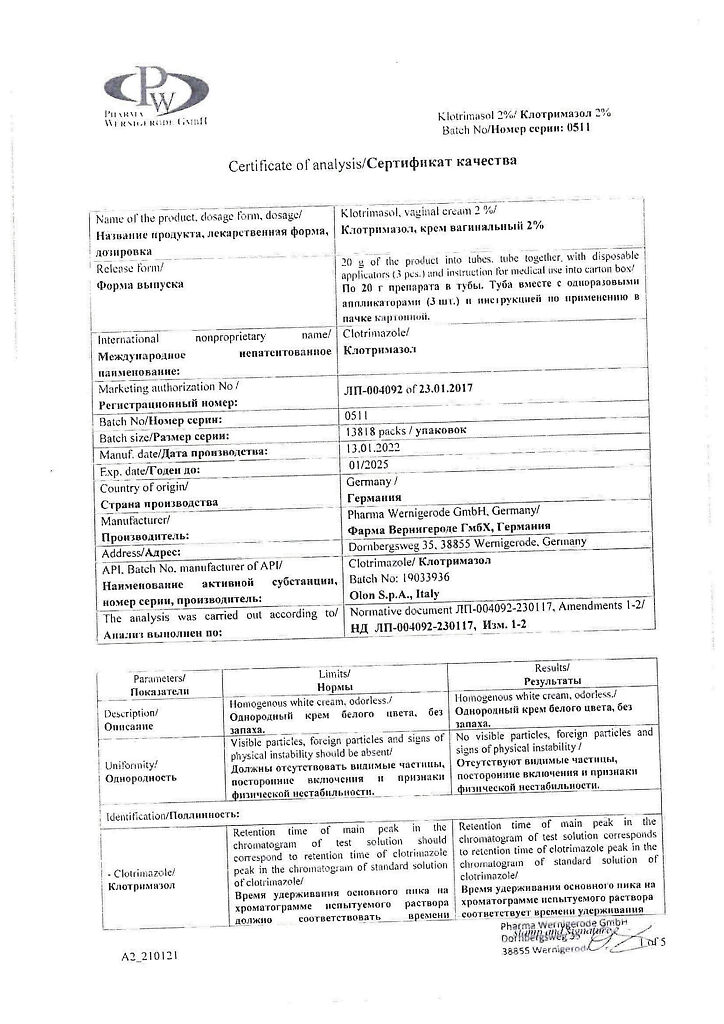
Pharma Wernigerode GmbH, Germany
Additional information
| Shelf life | 3 years. |
|---|---|
| Conditions of storage | At a temperature not exceeding 25 ° C. Keep out of reach of children. |
| Manufacturer | Pharma Wernigerode GmbH, Germany |
| Medication form | vaginal cream |
| Brand | Pharma Wernigerode GmbH |
Other forms…
Related products
Buy Clotrimazole, vaginal cream 2% 20 g with delivery to USA, UK, Europe and over 120 other countries.