No products in the cart.
Clopidogrel, 75 mg 28 pcs
€1.00
Out of stock
(E-mail when Stock is available)
Description
Pharmacodynamics
Clopidogrel is a specific and active platelet aggregation inhibitor; has coronary dilation action. Selectively reduces the binding of ADP to receptors on platelets and activation of GPI Ib/IIIa receptors under the action of ADP, weakening platelet aggregation.
Decreases platelet aggregation caused by other agonists by preventing their activation by released ADP, does not affect phosphodiesterase (PDE) activity. Irreversibly binds to platelet ADP receptors, which remain immune to ADP stimulation during the life cycle (about 7 days).
Inhibition of platelet aggregation is observed 2 hours after administration (40% inhibition) of the initial dose of 400 mg. The maximum effect (60% inhibition of aggregation) develops after 4 – 7 days of continuous use at a dose of 50 – 100 mg/day. The antiaggregant effect lasts for the entire period of platelet life (7 – 10 days).
In the presence of atherosclerotic vascular lesions it prevents the development of atherothrombosis regardless of the localization of the vascular process (cerebrovascular, cardiovascular or peripheral lesions).
Pharmacokinetics
Clopidogrel is rapidly absorbed when taken orally at 75 mg per day. Bioavailability is high. However, the concentration of the initial substance in plasma is low and does not reach the limit of measurement (0.025 mcg/L) as early as 2 hours after intake. Blood plasma protein binding is 98-94%.
Clopidogrel is rapidly metabolized in the liver. Its main metabolite – an inactive carboxylic acid derivative, which reaches its maximum concentration time (Tcmax), after repeated oral doses of 75 mg, in 1 hour (Tmax – about 3 mg/l).
About 50% of the drug is excreted by the kidneys and about 46% in the feces within 120 hours after administration. Half-life of the main metabolite after single and repeated administration is 8 hours. Concentrations of metabolites excreted by the kidneys are 50%.
Plasma concentrations of the main metabolite after 75 mg/day administration are lower in patients with severe kidney disease (creatinine clearance (CK) of 5-15 ml/min) compared to patients with moderate kidney disease (CK of 30 to 60 ml/min) and healthy individuals.
Indications
Indications
Prevention of atherothrombotic events in patients with myocardial infarction, ischemic stroke or with diagnosed peripheral artery occlusive disease.
Prevention of atherothrombotic events (in combination with acetylsalicylic acid) in patients with acute coronary syndrome:
-without ST-segment elevation (unstable angina or myocardial infarction without Q-wave), including patients who have had stenting for percutaneous coronary intervention;
-c ST-segment elevation (acute myocardial infarction) with medication-assisted treatment and thrombolysis options.
Active ingredient
Active ingredient
Composition
Composition
1 film-coated tablet contains:
the active substance:
clopidogrel hydrosulfate 97.875 mg, pose to clopidogrel – 75 mg;
excipients:
Pregelatinized starch,
anhydrous lactose (anhydrous milk sugar),
Macrogol (polyethylene glycol 6000),
magnesium stearate,
Microcrystalline cellulose (pH 112),
castor oil hydrogenated;
composition of the film coating:
Selecoat AQ-01673 (hypromellose (hydroxypropyl methylcellulose), macrogol (polyethylene glycol 400), macrogol (polyethylene glycol 6000), titanium dioxide, Ponso 4 R dye-based aluminum varnish)
How to take, the dosage
How to take, the dosage
Clopidogrel is taken orally, regardless of meals.
For prevention of ischemic disorders in patients after myocardial infarction, ischemic stroke and diagnosed peripheral arterial disease – 75 mg once daily. The treatment should be started within several days to 35 days after myocardial infarction and from 7 days to 6 months after ischemic stroke.
In acute coronary syndrome without ST-segment elevation (unstable angina pectoris or myocardial infarction without Q-wave), the treatment should be started with a single loading dose of 300 mg and then continued with 75 mg once daily (with simultaneous administration of acetylsalicylic acid 75-325 mg/day). Since the use of acetylsalicylic acid in high doses is associated with a high risk of bleeding, the recommended dose should not exceed 100 mg. The course of treatment is up to 1 year.
In acute myocardial infarction with ST-segment elevation, the drug is administered in a dose of 75 mg once daily with an initial loading dose in combination with acetylsalicylic acid with or without thrombolytics. For patients aged over 75 years, treatment with clopidogrel should be performed without using a loading dose. Combination therapy should be started as soon as possible after the onset of symptoms and continued for at least 4 weeks.
Interaction
Interaction
Warfarin: concomitant use with clopidogrel may increase the intensity of bleeding, so this combination is not recommended.
IIb/IIIa-receptor blockers: Administration of IIb/IIIa-receptor blockers together with clopidogrel requires caution in patients with increased risk of bleeding (in trauma and surgery or other pathological conditions).
Acetylsalicylic acid: Acetylsalicylic acid does not alter the effect of clopidogrel inhibiting ADP-induced platelet aggregation, but clopidogrel potentiates the effect of acetylsalicylic acid on collagen-induced platelet aggregation. However, concomitant administration of acetylsalicylic acid at 500 mg twice daily for 1 day with clopidogrel did not significantly increase the bleeding time caused by clopidogrel administration. There may be pharmacodynamic interaction between clopidogrel and acetylsalicylic acid, which leads to an increased risk of bleeding. Therefore, caution should be exercised when using them concomitantly. Although in clinical trials, patients received combined therapy with clopidogrel and acetylsalicylic acid for up to one year.
Heparin: According to the clinical trial conducted with the participation of healthy subjects when taking clopidogrel no change in the dose of heparin was required and its anticoagulant effect was not changed. Concomitant use of heparin did not alter the antiaggregant effect of clopidogrel. There is possible pharmacodynamic interaction between clopidogrel and heparin, which may increase the risk of bleeding, therefore concomitant use of these drugs requires caution.
Trombolytics: safety of concomitant use of clopidogrel, fibrin specific or fibrin specific drugs and heparin has been studied in patients with acute myocardial infarction. The frequency of clinically significant bleeding was similar to that observed when thrombolytics and heparin were used together with acetylsalicylic acid.
Non-steroidal anti-inflammatory drugs (NSAIDs): In a clinical study involving healthy volunteers, coadministration of clopidogrel and naproxen increased latent blood loss through the GI tract. However, due to the lack of studies on the interaction of clopidogrel with other NSAIDs, it is currently not known whether there is an increased risk of gastrointestinal bleeding when taking clopidogrel with other NSAIDs. Therefore, the use of NSAIDs, including COX-2 inhibitors, in combination with clopidogrel should be used with caution. Other combination therapy: since clopidogrel is metabolized to form its active metabolite partly by the CYP2C19 system, the use of drugs that inhibit this system (e.g., omeprazole) is not recommended.
A number of clinical studies with clopidogrel and other concomitantly administered drugs have been conducted to investigate possible pharmacodynamic pharmacokinetic interactions, which showed that:
Special Instructions
Special Instructions
During treatment it is necessary to monitor the parameters of the hemostatic system (activated partial thromboplastin time (APT), platelet count, tests of platelet functional activity); regular examination of liver functional activity.
Patients should be closely monitored for any signs of bleeding, including hidden bleeding, especially during the first weeks of use of the drug and/or after invasive cardiac procedures or surgery.
. Clopidogrel should be used with caution in patients with increased risk of bleeding in trauma, surgery, other pathological conditions, in diseases predisposing to the development of bleeding (especially gastrointestinal and ocular), as well as in patients receiving ASA, nonsteroidal anti-inflammatory drugs (including COX-2 inhibitors), heparin or glycoprotein IIb/IIIa inhibitors.
The concomitant use of clopidogrel and warfarin is not recommended because it may increase the intensity of bleeding (except in special rare clinical situations).
In case of surgical interventions, if antiaggregant effect is undesirable, the treatment should be discontinued 7 days before surgery.
Clopidogrel prolongs bleeding time and should be used with caution in patients with diseases predisposed to bleeding (especially gastrointestinal and intraocular).
Patients should be warned that since it takes longer to stop bleeding when using clopidogrel (with or without ASA), they should inform their physician about each bleeding event. Patients should also inform their physician (including the dentist) about taking the drug if they are going to have surgical interventions or dental procedures, and before starting any new medication.
Very rarely, thrombotic thrombocytopenic purpura (TTP) has been observed after taking clopidogrel (including short-term use). This condition is characterized by thrombocytopenia and microangiopathic hemolytic anemia combined with neurologic signs, renal dysfunction, or fever. TTP is a potentially life-threatening condition requiring immediate treatment, including plasmapheresis.
The experience with clopidogrel in patients with impaired renal function is limited; therefore, clopidogrel should be administered with caution in these patients.
In severe hepatic impairment, the risk of hemorrhagic diathesis should be kept in mind; experience with the drug in patients with moderate hepatic impairment is limited, so clopidogrel should be prescribed with caution in these patients.
Clopidogrel should not be used in patients with rare hereditary intolerance to galactose, lactase deficiency and glucose-galactose malabsorption syndrome.
Impact on ability to drive, other machinery.
Due to the possibility of dizziness when taking clopidogrel, caution should be exercised when driving and engaging in other potentially dangerous activities requiring increased concentration and rapid psychomotor reactions.
Contraindications
Contraindications
With caution:
Moderate hepatic insufficiency, chronic renal failure (CKD), pathological conditions that increase the risk of bleeding (including trauma, surgery), simultaneous use of ASA, warfarin, nonsteroidal anti-inflammatory drugs (NSAIDs) (including COX-2 inhibitors), heparin and glycoprotein IIb/IIIa inhibitors, inherited reduced function of CYP2C19 isoenzyme.
Side effects
Side effects
Blood coagulation system: often – bleeding (in most cases – during the first month of treatment), purpura, hematoma; infrequently – conjunctival bleeding; rarely – intracranial bleeding; prolongation of bleeding time, leukopenia, decreased neutrophil count and eosinophilia, reduced platelet count.
Blood system disorders: very rarely – thrombocytopenic thrombohemolytic purpura, severe thrombocytopenia (platelet count < 30 000/μl), granulocytopenia, agranulocytosis, anemia including aplastic anemia, pancytopenia.
Nervous system: infrequent headache, dizziness, paresthesia, rarely vertigo; very rare – mental confusion, hallucinations.
Cardiovascular system: frequently – hematoma; very rarely – severe bleeding, bleeding from the operating wound, vasculitis, decreased blood pressure.
Respiratory system: very common – nasal bleeding; very rare – bronchospasm, interstitial pneumonitis, pulmonary bleeding, hemoptysis.
The digestive system: frequently – diarrhea, abdominal pain, dyspepsia, gastrointestinal bleeding; infrequently – gastric and duodenal ulcer, gastritis, vomiting, nausea, flatulence, constipation; rarely – retroperitoneal bleeding; very rarely – colitis (including ulcerative or lymphatic).ulcerative or lymphocytic), pancreatitis, change in sense of taste, stomatitis, hepatitis, acute liver failure, increased liver enzymes activity,
Musculoskeletal system: very rare – arthralgia, arthritis, myalgia.
Urinary system: infrequent – hematuria; very rare – glomerulonephritis, hypercreatininemia.
Dermatological reactions: very rarely – bullous rash (erythema multiforme, Stevens-Johnson syndrome, toxic epidermal necrolysis), erythematous rash, eczema, lichen planus.
Allergic reactions: very rare – angioedema, urticaria, anaphylactoid reactions, serum sickness.
Other: very rarely – increase in body temperature.
Overdose
Overdose
Clopidogrel overdose may lead to prolonged bleeding time and hemorrhagic complications. If bleeding is detected, appropriate treatment should be applied.
No antidotes to the pharmaceutical activity of clopidogrel have been identified.
If rapid correction of prolonged bleeding time is necessary, platelet transfusion is recommended.
Pregnancy use
Pregnancy use
Due to lack of data, it is not recommended to take clopidogrel during pregnancy and lactation.
Similarities
Similarities
Additional information
| Shelf life | 2 years |
|---|---|
| Conditions of storage | In a dry, light-protected place at a temperature not exceeding 25 °C |
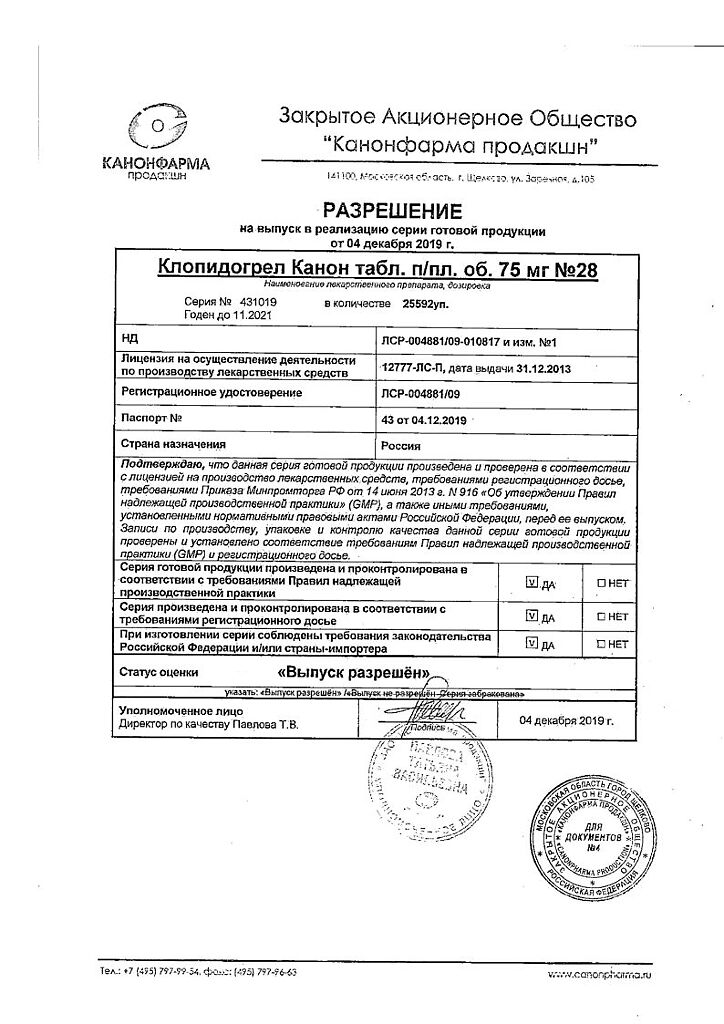
| Manufacturer | Kanonfarma Production ZAO, Russia |
| Medication form | pills |
| Brand | Kanonfarma Production ZAO |
Other forms…
Related products
Buy Clopidogrel, 75 mg 28 pcs with delivery to USA, UK, Europe and over 120 other countries.