No products in the cart.
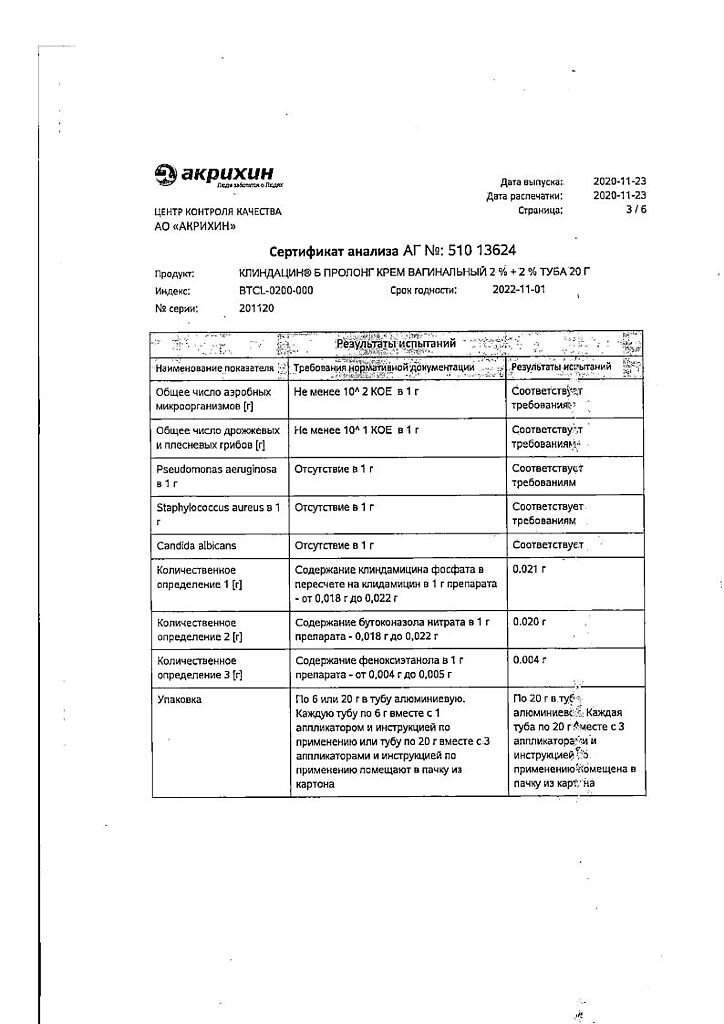
Clindacin B prolong, vaginal cream 2%+2% 20 g
€17.87 €14.89
Description
Pharmacotherapeutic group: Antimicrobial and antifungal agent.
Pharmacological action
Pharmacodynamics
Butoconazole – Imidazole derivative, has fungicidal activity against Candida fungi, Trichophyton, Microsporum, Epidermaphyton and some Gram-positive bacteria. It is most effective against candidiasis. By blocking in the cell membrane formation of ergosterol from lanosterol it increases membrane permeability, which leads to lysis of the fungus cell.
Clindamycin is a bacteriostatic antibiotic of the lincosamide group, it has a broad spectrum of action, binds to 50S subunit of ribosomal membrane and inhibits protein synthesis in a microbial cell. Bactericidal action is possible against a number of Gram-positive cocci. Under in vitro conditions the following microorganisms causing bacterial vaginosis are sensitive to clindamycin: Gardnerella vaginalis, Mobiluncus spp., Mycoplasma hominis, Bacteroides spp., Peptostreptococcus spp.
The hydrophilic cream base provides a gel-like consistency of the drug at 35-40 ° C. The cream does not melt when applied intravaginally, which ensures that the active substances remain on the vaginal mucosa for 1-3 days. There is a cross-resistance between clindamycin and lincomycin.
Pharmacokinetics
Butoconazole
In intravaginal administration, approximately 1.7% of the administered dose of butoconazole. Cmax in plasma butoconazole is reached after 13 hours and is 2-18.6 ng/ml. Butoconazole is extensively metabolized, partially excreted by the kidneys and intestine.
Clindamycin
absorption
. After use of clindamycin intravaginally at a dose of 100 mg/day once (as 2% clindamycin phosphate cream) for 7 days, the Cmax of clindamycin in plasma is reached after 10 h (4-24 h) and averages 18 ng/mL (4-47 ng/mL) on day 1 and 25 ng/mL (6-61 ng/mL) on day 7, with systemic absorption of about 4% (06-11%) of the administered dose.
In women with bacterial vaginosis, approximately 4% of clindamycin undergoes systemic absorption (with a smaller variation of 2-8%) with a similar dosing regimen, Cmax is reached 14 h (4-24 h) after administration and
averages 13 ng/mL (6-34 ng/mL) on day 1 and 16 ng/mL (7-26 ng/mL) on day 7. The systemic effects of clindamycin are less pronounced with intravaginal administration than with oral or IV administration.
Evolution
The T1/2 is 1.5-2.6 h. Clindamycin almost does not cumulate after repeated intravaginal administration.
Pharmacokinetics in Special Patient Groups
Not enough patients aged 65 years or older have participated in clinical trials of clindamycin 2% vaginal cream to allow assessment of the difference in clinical response to therapy between this age group and younger patients. In the available reports from clinical experience, no difference in response between older and younger patients was observed.
Indications
Indications
Active ingredient
Active ingredient
Composition
Composition
Vaginal cream from almost white to white with a grayish tint, with a specific smell.
Associates: Preservative Euxyl PE 9010 (phenoxyethanol 90%, ethylhexylglycerol 10%) – 0.5 g, which is equivalent to 0.45 g of phenoxyethanol, propylene glycol – 5 g, isopropyl myristate – 8 g, macrogoal cetostearate (macrogoal-20 cetostearyl ether) – 2 g, cetostearyl alcohol (cetyl alcohol 60%, stearyl alcohol 40%) – 6 g, hydroxypropyl diacrystalline phosphate – 8 g, sodium hydroxide – 0.26 g, purified water – up to 100 g.
How to take, the dosage
How to take, the dosage
Interaction
Interaction
Special Instructions
Special Instructions
Contraindications
Contraindications
With caution:allergic diseases, concomitant use of muscle relaxants.
Side effects
Side effects
Overdose
Overdose
Additional information
| Weight | 0.060 kg |
|---|---|
| Shelf life | 2 years. |
| Conditions of storage | The drug should be kept out of reach of children at 15°C to 25°C. |
| Manufacturer | Akrihin HFC JSC, Russia |
| Medication form | vaginal cream |
| Brand | Akrihin HFC JSC |
Related products
Gynecology and Obstetrics
Buy Clindacin B prolong, vaginal cream 2%+2% 20 g with delivery to USA, UK, Europe and over 120 other countries.