No products in the cart.
Citramon P Reneval, tablets 10 pcs
€4.00 €3.64
Description
- moderate pain syndrome of various etiologies: headache, migraine, toothache, neuralgia, arthralgia, myalgia, algodysmenorrhea;
- fever conditions with acute respiratory infections, influenza and rheumatic diseases.
.
Indications
Indications
moderately severe pain syndrome of various etiologies: headache, migraine, toothache, neuralgia, arthralgia, myalgia, algomenorrhea;
febrile conditions during acute respiratory viral infections, influenza and rheumatic diseases.
Special instructions
Special instructions
The drug should be prescribed with caution to patients with bronchial asthma and gout.
It is not recommended to prescribe the drug to children, especially with chickenpox and influenza, due to the high risk of developing Reye’s syndrome.
Citramon should not be prescribed simultaneously with barbiturates, anticonvulsants, salicylates, and rifampicin.
Patients taking Citramon should refrain from drinking alcohol.
If it is necessary to prescribe a drug during pregnancy, the expected benefit to the mother should be balanced against the potential risk to the fetus. If short-term administration of Citramon is necessary during lactation, cessation of breastfeeding is not required.
Active ingredient
Active ingredient
Acetylsalicylic acid, Caffeine, Paracetamol
Composition
Composition
1 tablet contains:
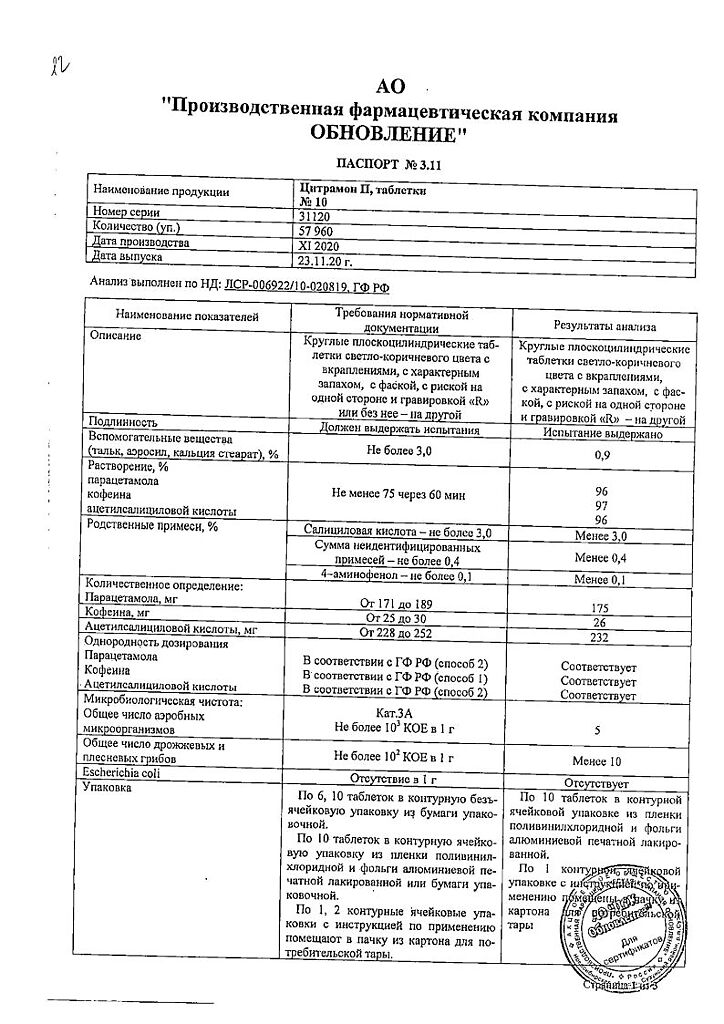
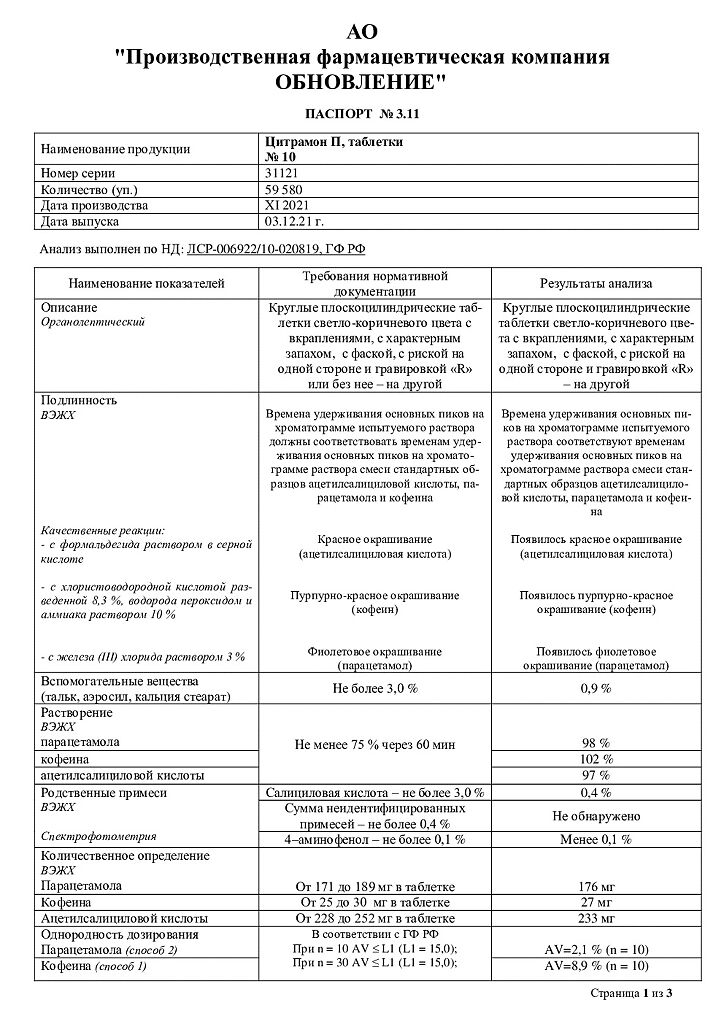
active ingredient: acetylsalicylic acid 240 mg, paracetamol 180 mg, caffeine 30 mg
Contraindications
Contraindications
hypersensitivity;
erosive and ulcerative lesions of the gastrointestinal tract (in the acute phase);
gastrointestinal bleeding;
“aspirin” asthma;
hemophilia;
hemorrhagic diathesis;
hypoprothrombinemia;
portal hypertension;
vitamin deficiency K;
renal failure;
deficiency of glucose-6-phosphate dehydrogenase;
severe arterial hypertension;
severe course of ischemic heart disease;
glaucoma;
increased excitability;
sleep disorders;
surgical interventions accompanied by bleeding.
With caution: gout, liver disease.
Side Effects
Side Effects
On the part of the immune system: hypersensitivity reactions, including anaphylaxis, anaphylactic shock, rhinitis, nasal congestion.
From the skin and subcutaneous tissue: itching, rash on the skin and mucous membranes, including erythematous rashes; urticaria, angioedema, erythema multiforme exudative, Stevens-Johnson syndrome, toxic epidermal necrolysis.
Gastrointestinal disorders: dyspeptic disorders, including nausea, vomiting, discomfort and epigastric pain, heartburn, abdominal pain; inflammation of the gastrointestinal tract, erosive and ulcerative lesions of the gastrointestinal tract, which can in rare cases cause gastrointestinal bleeding and perforation with corresponding laboratory and clinical manifestations.
Hepatobiliary disorders: increased activity of liver enzymes, usually without the development of jaundice, hepatonecrosis (dose-dependent effect).
Endocrine disorders: hypoglycemia, up to hypoglycemic coma.
Neurological disorders: headache, dizziness, tremor, paresthesia, fear, anxiety, agitation, irritability, sleep disturbance, insomnia, anxiety, general weakness, ringing in the ears.
Cardiac disorders: tachycardia, palpitations, arterial hypertension.
On the part of the blood and lymphatic system: anemia, sulfate hemoglobinemia and methemoglobinemia (cyanosis, shortness of breath, pain in the heart), hemolytic anemia, due to the antiplatelet effect on platelets, acetylsalicylic acid may increase the risk of bleeding. Bleeding events such as intraoperative hemorrhages, hematomas, bleeding from the genitourinary system, nosebleeds, bleeding from the gums, gastrointestinal bleeding and cerebral hemorrhages have been observed.
Others: bleeding can lead to acute and chronic posthemorrhagic anemia/iron deficiency anemia (due to the so-called hidden microbleeding) with corresponding laboratory manifestations and clinical symptoms, such as asthenia, pale skin, hypoperfusion; non-cardiogenic pulmonary edema.
Interaction
Interaction
Enhances the effect of heparin, indirect anticoagulants, reserpine, steroid hormones and hypoglycemic drugs.
Concomitant use with other NSAIDs and methotrexate increases the risk of side effects.
Reduces the effectiveness of spironolactone, furosemide, antihypertensive drugs, as well as anti-gout drugs that promote the excretion of uric acid.
Barbiturates, rifampicin, salicylamide, antiepileptic drugs and other stimulants of microsomal oxidation contribute to the formation of toxic paracetamol metabolites that affect liver function.
Metoclopramide accelerates the absorption of paracetamol.
Under the influence of paracetamol, T1/2 of chloramphenicol increases 5 times. When taken repeatedly, paracetamol may enhance the effect of anticoagulants (dicoumarin derivatives).
The simultaneous use of paracetamol and ethanol increases the risk of developing hepatotoxic effects.
Caffeine accelerates the absorption of ergotamine.
Overdose
Overdose
Symptoms (due to acetylsalicylic acid): for mild intoxication – nausea, vomiting, gastralgia, dizziness, ringing in the ears; severe intoxication – lethargy, drowsiness, collapse, convulsions, bronchospasm, difficulty breathing, anuria, bleeding. Initially, central hyperventilation of the lungs leads to respiratory alkalosis (shortness of breath, suffocation, cyanosis, perspiration). As intoxication increases, progressive respiratory paralysis and uncoupling of oxidative phosphorylation cause respiratory acidosis.
Treatment: constant monitoring of acid-base balance and electrolyte balance; depending on the state of metabolism – the introduction of sodium bicarbonate, sodium citrate or sodium lactate. Increasing reserve alkalinity enhances the excretion of acetylsalicylic acid due to alkalinization of urine.
Storage conditions
Storage conditions
Store out of the reach of children in the original packaging at a temperature not exceeding 25 °C.
Shelf life
Shelf life
5 years.
Manufacturer
Manufacturer
Update of PFC JSC, Russia
Additional information
| Shelf life | 5 years. |
|---|---|
| Conditions of storage | Keep out of reach of children in the original packaging at a temperature not exceeding 25 ° C. |
| Manufacturer | Update PFC AO, Russia |
| Medication form | pills |
| Brand | Update PFC AO |
Related products
Buy Citramon P Reneval, tablets 10 pcs with delivery to USA, UK, Europe and over 120 other countries.