No products in the cart.
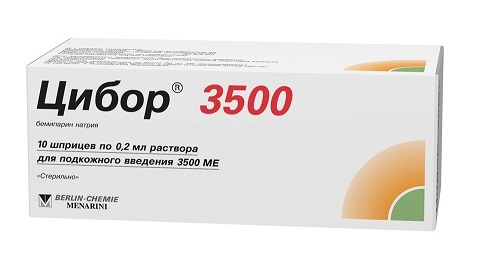
Cibor,3500 me 0,2 ml syringes 10 pcs
€102.25 €88.61
Description
Cibor is an anticoagulant drug for parenteral administration. The drug contains the active component – bemiparin sodium – a low molecular weight heparin, which is obtained by depolymerization of sodium heparin extracted from the mucous membrane of pig intestine. The average molecular weight of bemiparin is 3600Da.
The content of molecular chains with a molar mass of less than 2000Da is less than 35%, with a molar mass of 2000-6000Da – 50-75%, with a molar mass of more than 6000Da – less than 15%.
Bemiparin sodium has anti-Xa-factor activity of about 80-120Me of anti-Xa per 1mg of dry matter. Anti-IIa-factor activity is 5-20Me of anti-IIa-factor per 1mg of dry sodium bemiparin. The ratio of anti-Xa-factor and anti-IIa-factor activity is about 8. Cibor has a pronounced anticoagulation effect; when used in therapeutic doses it practically does not increase the clotting time.
When administered subcutaneously the bioavailability is 96%. Maximal activity is observed 2-4 hours after injection. When administering therapeutic doses the anti-IIa-factor activity is practically not manifested.
The elimination half-life with doses of 2500 to 12500 ME reaches 5-6 hours. To achieve the therapeutic effect it is sufficient to administer the drug once a day.
Indications
Indications
The drug Cibor is intended for the prevention of blood clotting in the extracorporeal circulation system during a hemodialysis session.
In addition, the drug Cibor 2500 is used to prevent thromboembolism during general surgical interventions.
The drug Cibor 3500 is also used for the prevention of thromboembolism during surgical interventions in orthopedic practice.
Pharmacological effect
Pharmacological effect
Cibor is an anticoagulant drug for parenteral administration. The drug contains the active component – bemiparin sodium – low molecular weight heparin, which is obtained by depolymerization of sodium heparin isolated from the mucous membrane of the pig intestine. The average molecular weight of bemiparin is 3600 Da.
The content of molecular chains with a molar mass of less than 2000 Da is less than 35%, with a molar mass of 2000-6000 Da – 50-75%, with a molar mass of more than 6000 Da – less than 15%.
Bemiparin sodium has anti-factor Xa activity of about 80-120 IU anti-factor Xa per 1 mg of dry matter. Anti-IIa factor activity – 5-20 IU of anti-factor IIa per 1 mg of dry bemiparin sodium. The ratio of anti-Xa factor and anti-IIa factor activity is about 8. Cibor has a pronounced anticoagulant effect; when used in therapeutic doses, it practically does not increase blood clotting time.
When administered subcutaneously, bioavailability is 96%. Maximum activity is observed 2-4 hours after injection. When therapeutic doses are administered, anti-IIa factor activity is practically not observed.
The half-life when using a dose from 2500 to 12500 IU reaches 5-6 hours. To achieve a therapeutic effect, it is sufficient to administer the drug once a day.
Active ingredient
Active ingredient
Bemiparin sodium
Composition
Composition
1 ml of injection solution Cibor 3500 contains:
Bemiparin sodium (equivalent to antifactor-Xa) – 17500 IU;
Additional substances.
Solution for injection in ready-made syringes of 0.2 ml, 2 syringes packed in a blister, 1, 5, 15, 20 or 50 blisters placed in a cardboard box.
Pregnancy
Pregnancy
Cibor during pregnancy is prescribed after a careful assessment of the risk/benefit ratio. In animal studies, the drug Tzibor did not have a teratogenic effect; there is no data on the penetration of bemiparin through the hematoplacental barrier.
If it is necessary to use bemiparin during lactation, breastfeeding should be interrupted after consulting with your doctor.
Contraindications
Contraindications
Cibor is not prescribed to patients with hypersensitivity to bemiparin and heparin, as well as to patients with thrombocytopenia immunologically caused by heparin (including a history or suspicion of such a condition).
The drug should not be prescribed to persons suffering from blood coagulation disorders with a risk of bleeding, severe dysfunction of the pancreas and liver, as well as to persons with active bleeding.
Bemiparin is not prescribed for acute endocarditis of bacterial etiology, chronic endocarditis, injuries or surgical interventions in the area of vision, hearing and the central nervous system, for patients with disseminated intravascular coagulation syndrome.
It is not recommended to use the drug in persons with a high risk of bleeding, in particular with active peptic ulcers, cerebral aneurysms, hemorrhagic stroke and brain tumors.
Cibor is not used in pediatrics.
Bemiparin is prescribed with caution to patients suffering from impaired liver and kidney function, thrombocytopenia, circulatory disorders of the iris and retina of vascular origin, uncontrolled arterial hypertension, urolithiasis, as well as to persons with a history of ulcerative lesions of the mucous membrane of the stomach and duodenum.
Due to the increased risk of hyperkalemia, Zibor should be administered with caution to patients with diabetes mellitus, metabolic acidosis, chronic renal failure and elevated plasma potassium levels, as well as to patients receiving therapy with potassium-sparing diuretics (such patients should have their potassium level determined before starting bemiparin therapy and monitored throughout the course of treatment).
Particular care should be taken when performing lumbar puncture, epidural or spinal anesthesia in patients receiving bemiparin due to the risk of developing a spinal or epidural hematoma, which may result in permanent paralysis. The interval between taking bemiparin and performing these manipulations is determined by the attending physician. If symptoms of an epidural or spinal hematoma appear, diagnosis should be made immediately and appropriate therapy should be prescribed.
Side Effects
Side Effects
The following undesirable effects may develop when using the drug Cibor:
Liver failure: transient increase in the activity of liver enzymes.
From the hematopoietic system: thrombocytopenia of varying severity, type I or II. If thrombocytopenia type I develops, discontinuation of the drug is not required. If, with the development of type II thrombocytopenia (usually observed on days 5-21 of bemiparin therapy), the platelet count decreases by 30-50% (compared to the level before the start of therapy), bemiparin should be discontinued and alternative therapy should be prescribed.
Allergic reactions: urticaria, skin itching, anaphylactoid reactions. In isolated cases, the development of skin necrosis was noted, which was preceded by erythema and erythematous painful spots; with the development of such side effects, immediate discontinuation of bemiparin is required.
Local reactions: ecchymosis, necrosis, pain and hematoma at the injection site.
Others: bleeding, including from the skin, wounds, digestive and genitourinary tracts, mucous membranes. Osteoporosis. Epidural and spinal hematoma during lumbar puncture, spinal or epidural anesthesia.
Interaction
Interaction
There are no data on the pharmacological interactions of bemiparin; possible interactions should be taken into account based on data on other low molecular weight heparins, as follows:
Bemiparin should not be used in combination with other drugs that have anticoagulant properties and can inhibit platelet aggregation, as well as with systemic glucocorticosteroid drugs and dextran, as these combinations increase the risk of bleeding. If the combined use of these drugs cannot be avoided, blood clotting and the patient’s condition should be carefully monitored.
Bemiparin should also be used with caution in combination with drugs that contribute to the development of hyperkalemia.
With the simultaneous use of the drug Cibor with nitroglycerin for intravenous administration, the effectiveness of bemiparin sodium may be reduced.
It is prohibited to mix the solution with other drugs for parenteral use.
Overdose
Overdose
When using excessive doses of the drug Cibor, patients experienced the development of bleeding.
Treatment is prescribed depending on the severity of hemorrhage, as well as the risk of thrombosis. For minor bleeding, therapy is rarely required. For more serious hemorrhages, administration of protamine sulfate is prescribed, which slightly reduces the anti-factor Xa activity of the drug Cibor for 2 hours.
For intravenous administration, the dose of protamine sulfate is calculated individually depending on the dose of bemiparin (1.4 mg of protamine sulfate per 100 IU of antifactor-Xa).
Storage conditions
Storage conditions
At a temperature not exceeding 30 °C (do not freeze)
Shelf life
Shelf life
2 years
Manufacturer
Manufacturer
ROVI Contract Manufacturing S.L., Spain
Additional information
| Shelf life | 2 years |
|---|---|
| Conditions of storage | At a temperature not exceeding 30 °C (do not freeze) |
| Manufacturer | ROVI Contract Manufechering S.L., Spain |
| Medication form | solution for injection |
| Brand | ROVI Contract Manufechering S.L. |
Related products
Buy Cibor,3500 me 0,2 ml syringes 10 pcs with delivery to USA, UK, Europe and over 120 other countries.