No products in the cart.
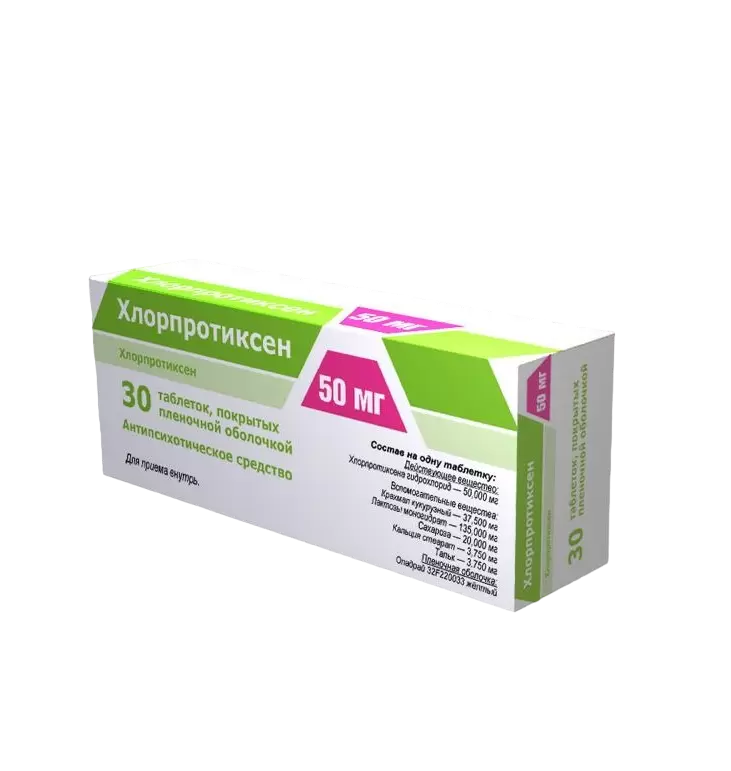
Chlorprotixen, 50 mg 30 pcs
€26.13 €22.64
Description
Chlorprotixene is a neuroleptic derivative of thioxanthene. It has antipsychotic, marked sedative and moderate antidepressant effect.
Antipsychotic effect of neuroleptics is associated with blockade of dopamine receptors, and possibly also blockade of 5-NT
(5-hydroxytryptamine) receptors. In vivo, chlorprotixene has a high affinity for dopamine receptors type D1 and D2. Chlorprotixene also has high affinity for 5-HT2 receptors, a1-adrenoceptors, histamine (H1) and cholinergic muscarinic receptors. The receptor binding profile of chlorprotixen is very similar to that of clozapine, but it has approximately 10 times higher affinity for dopamine receptors.
Chlorprotixen reduces or eliminates anxiety, obsessions, psychomotor agitation, anxiety, insomnia, as well as hallucinations, delusions and other psychotic symptoms. The very low incidence of extrapyramidal effects (about 1%) and tardive dyskinesia (about 0.05%) indicate that chlorprotixen can be successfully used for maintenance therapy of patients with psychotic disorders.
Low doses of chlorprotixen have an antidepressant effect, which makes the drug useful in mental disorders characterized by anxiety, depression and anxiety. Chlorprotixene therapy also reduces the severity of associated psychosomatic symptoms. Chlorprotixene is not addictive, addictive, or tolerant. In addition, chlorprotixene potentiates the effect of analgesics, has its own analgesic effect as well as antipruritic and antiemetic…
Pharmacokinetics
Absorption
When taken orally the maximum concentration in plasma is reached after about 2 hours (range 0.5-6 hours). The average bioavailability of Chlorprotixen when ingested is approximately 12% (range 5-32%).
Distribution
The apparent volume of distribution (Vd)β is approximately 15.5 L/kg. Binding to plasma proteins is over 99%.
Chlorprotixene penetrates the placental barrier.
Biotransformation
Metabolism of chlorprotixene is primarily by sulfoxidation and N-demethylation of the side chain. The hydroxylation of the ring and N-oxidation are expressed to a lesser extent. Chlorprotixene is determined in bile, indicating the presence of intestinal-hepatic circulation of the drug. Chlorprotixene metabolites lack neuroleptic activity.
Excretion
The elimination half-life is approximately 16 hours (range 4 – 33 hours). Mean systemic clearance (Cls) corresponds to approximately 1.2 L/min. Chlorprotixene is excreted with feces and urine.
In women who are breastfeeding, chlorprotixene is excreted in small amounts with milk. The maternal milk to plasma drug concentration ratio ranges from 1.2 to 2.6.
No differences in plasma concentrations or excretion rates were found between the control group and the alcohol dependent patient group, regardless of whether the latter were sober or acutely intoxicated during the study.
Indications
Indications
• Schizophrenia and other psychoses occurring with psychomotor agitation, agitation and anxiety.
• Withdrawal syndrome in alcoholism and drug addiction.
• Depressive states, neuroses, psychosomatic disorders with anxiety, tension, restlessness, insomnia, sleep disorders.
• Epilepsy and mental retardation, combined with mental disorders: agitation, agitation, mood lability and behavioral disorders.
• Pain (in combination with analgesics).
• Geriatrics: hyperactivity, agitation, irritability, confusion, anxiety, behavioral and sleep disorders.
Pharmacological effect
Pharmacological effect
Chlorprothixene is an antipsychotic drug derived from thioxanthene. It has antipsychotic, pronounced sedative and moderate antidepressant effects.
The antipsychotic effect of neuroleptics is associated with blockade of dopamine receptors, and also, possibly, blockade of 5-HT
(5-hydroxytryptamine) receptors. In vivo, chlorprothixene has a high affinity for dopamine receptors type D1 and D2. Chlorprothixene also has high affinity for 5-HT2 receptors, α1-adrenergic receptors, histamine (H1) and cholinergic muscarinic receptors. The receptor binding profile of chlorprothixene is very similar to that of clozapine, but it has approximately 10 times higher affinity for dopamine receptors.
Chlorprothixene reduces the severity or eliminates anxiety, obsessions, psychomotor agitation, restlessness, insomnia, as well as hallucinations, delusions and other psychotic symptoms. The very low incidence of extrapyramidal effects (about 1%) and tardive dyskinesia (about 0.05%) indicate that chlorprothixene can be successfully used for maintenance treatment of patients with psychotic disorders.
Low doses of chlorprothixene have an antidepressant effect, which makes the drug useful for mental disorders characterized by anxiety, depression and restlessness. Also, during therapy with chlorprothixene, the severity of associated psychosomatic symptoms decreases. Chlorprothixene does not cause addiction, dependence or tolerance. In addition, chlorprothixene potentiates the effect of analgesics, has its own analgesic effect, as well as antipruritic and antiemetic effects…
Pharmacokinetics
Suction
When administered orally, maximum plasma concentrations are achieved in approximately 2 hours (range 0.5-6 hours). The average oral bioavailability of chlorprothixene is approximately 12% (range 5-32%).
Distribution
The apparent volume of distribution (Vd)β is approximately 15.5 l/kg. Binding to plasma proteins is more than 99%.
Chlorprothixene penetrates the placental barrier.
Biotransformation
The metabolism of chlorprothixene occurs primarily through sulfoxidation and N-demethylation of the side chain. Ring hydroxylation and N-oxidation are less pronounced. Chlorprothixene is determined in bile, which indicates the presence of enterohepatic circulation of the drug. Chlorprothixene metabolites lack neuroleptic activity.
Removal
The half-life is approximately 16 hours (range 4 – 33 hours). The average systemic clearance (Cls) corresponds to approximately 1.2 l/min. Chlorprothixene is excreted in feces and urine.
In women who are breastfeeding, chlorprothixene is excreted in milk in small quantities. The ratio between the concentration of the drug in breast milk and blood plasma varies from 1.2 to 2.6.
There were no differences in plasma concentrations or elimination rates between the control group and the alcoholic group, regardless of whether the latter were sober or acutely intoxicated at the time of the study.
Active ingredient
Active ingredient
Chlorprothixene
Composition
Composition
One film-coated tablet, 50 mg contains:
Active ingredient:
Chlorprothixene hydrochloride – 50,000 mg
Excipients:
Corn starch – 37,500 mg
Lactose monohydrate – 135,000 mg
Sucrose – 20,000 mg
Calcium stearate – 3,750 mg
Talc – 3,750 mg
Film casing:
Opadry 32F220033 yellow – 7,500 mg
Pregnancy
Pregnancy
Pregnancy
Clinical experience in pregnant women is limited. Chlorprothixene should not be prescribed during pregnancy unless the expected benefit to the patient outweighs the possible risk to the fetus.
Neonates exposed to antipsychotics (including chlorprothixene) during the third trimester of pregnancy are at risk of adverse reactions, including extrapyramidal symptoms and/or withdrawal symptoms, which may vary in severity and duration after delivery. Cases of agitation, increased and decreased tone, tremors, drowsiness, respiratory distress, and eating disorders have been recorded. Therefore, newborns should be closely monitored.
Breastfeeding
Taking into account the fact that chlorprothixene is present in human milk in small concentrations, it is unlikely to have a negative effect on the child if the drug is prescribed to the mother in therapeutic doses. The dose administered orally to the child is approximately 2% of the daily maternal dose, adjusted for body weight. During treatment with Chlorprothixene, breastfeeding is allowed if deemed clinically necessary. However, it is recommended to monitor the condition of the newborn, especially in the first 4 weeks after birth.
Fertility
Adverse effects such as hyperprolactinemia, galactorrhea, amenorrhea, ejaculation disorders, and erectile dysfunction have been recorded in humans (see section “Side Effects”). These adverse reactions may have a negative impact on sexual function and fertility in women and/or men.
If clinically significant hyperprolactinemia, galactorrhea, amenorrhea or manifestations of sexual dysfunction occur, dosage reduction (if possible) or discontinuation of the drug should be considered. These side effects are reversible after discontinuation of the drug.
The potential effects of the drug on fertility have not been studied in animals.
Contraindications
Contraindications
• Hypersensitivity to chlorprothixene or any of the excipients.
• Hypersensitivity to drugs of the thioxanthene group.
• Vascular collapse, depression of consciousness of any origin (including those caused by alcohol, barbiturates or opiates), coma.
• Known uncorrectable hypokalemia or hypomagnesemia.
• Patient history of clinically significant cardiovascular disease (for example, bradycardia (heart rate less than 50 beats per minute), recent myocardial infarction, decompensated heart failure, cardiac hypertrophy, arrhythmias for which class IA and III antiarrhythmics are prescribed), ventricular arrhythmias or polymorphic ventricular tachycardia of the torsade de pointes type (Torsade de Points).
• Congenital long QT syndrome or acquired long QT interval (QTc over 450 ms in men and 470 ms in women).
• Concomitant use with drugs that significantly prolong the QT interval.
• Lactose or fructose intolerance, lactase deficiency, glucose-galactose malabsorption, sucrase/isomaltase deficiency (due to the presence of lactose and sucrose in the composition).
With caution
Organic diseases of the brain; mental retardation; a family history of relatives suffering from cardiovascular diseases, as well as cases of prolongation of the QT interval; seizure disorders; severe liver and kidney failure; a rare pathological condition in the form of a small anterior chamber of the eye and its narrow angle (possible development of attacks of acute glaucoma associated with pupil dilation); myasthenia gravis; benign prostatic hypertrophy; pheochromocytoma; prolactin-dependent neoplasms; severe arterial hypotension or orthostatic disorders; Parkinson’s disease; diseases of the hematopoietic system; hyperthyroidism; painful urination, urinary retention; pyloric stenosis; intestinal obstruction; presence of risk factors for stroke; diabetes mellitus; abuse of opiates and alcohol; pregnancy, breastfeeding period; children and adolescents under 18 years of age (due to the lack of strictly controlled studies).
Side Effects
Side Effects
Drowsiness, tachycardia, dry mouth, increased sweating, difficulty in accommodation. These side effects, which usually occur early in therapy, often disappear as therapy continues. Orthostatic hypotension may occur, especially when using Chlorprothixene in high dosages.
Dizziness, dysmenorrhea, skin rashes, and constipation are rare. Extrapyramidal symptoms are especially rare. Isolated cases of a decrease in the seizure threshold, the occurrence of transient benign leukopenia and hemolytic anemia have been described.
With long-term use, especially in high doses, the following may be observed: cholestatic jaundice, galactorrhea, gynecomastia, decreased potency and/or libido, increased appetite, increased body weight.
Interaction
Interaction
Chlorprothixene may enhance the sedative effect of alcohol, the effect of barbiturates and other CNS depressants.
Chlorprothixene should not be prescribed together with guanethidine and similarly active drugs, since antipsychotics can enhance or weaken the effect of antihypertensive drugs; the antihypertensive effect of guanethidine and similarly active drugs is reduced.
Concomitant use of antipsychotics and lithium drugs increases the risk of neurotoxicity.
Tricyclic antidepressants and antipsychotics mutually inhibit each other’s metabolism.
Chlorprothixene may reduce the effectiveness of levodopa and the effect of adrenergic drugs.
The simultaneous use of Chlorprothixene and drugs with established anticholinergic effects enhances their anticholinergic effects.
Concomitant use with metoclopramide and piperazine increases the risk of developing extrapyramidal disorders.
The antihistamine effect of chlorprothixene may suppress or eliminate the alcohol/disulfiram reaction.
The increase in the QT interval on the electrocardiogram, characteristic of therapy with antipsychotic drugs, can be enhanced by concomitant use of drugs that significantly prolong the QT interval: class IA and III antiarrhythmic drugs (quinidine, amiodarone, sotalol, dofetilide), some antipsychotics (thioridazine), some macrolide antibiotics (erythromycin) and quinolone antibiotics (gatifloxacin, moxifloxacin), some antihistamines (terfenadine, astemizole), as well as cisapride, lithium and other drugs that significantly increase the QT interval. The simultaneous use of Chlorprothixene and the above drugs should be avoided. Chlorprothixene should be used with caution concurrently with drugs that cause electrolyte disturbances (thiazide and thiazide-like diuretics) and drugs that can increase plasma concentrations of chlorprothixene due to a possible increased risk of QT prolongation and life-threatening arrhythmias.
Antipsychotics are metabolized by hepatic isoenzymes of the cytochrome P450 system. Drugs that inhibit the 2D6 isoenzyme (eg, paroxetine, fluoxetine, chloramphenicol, disulfiram, isoniazid, monoamine oxidase inhibitors, oral contraceptives, and, to a lesser extent, buspirone, sertraline and citalopram) may increase plasma levels of chlorprothixene.
Manufacturer
Manufacturer
Pharmproject, Russia
Additional information
| Manufacturer | Pharmproject, Russia |
|---|---|
| Medication form | pills |
| Brand | Pharmproject |
Other forms…
Related products
Buy Chlorprotixen, 50 mg 30 pcs with delivery to USA, UK, Europe and over 120 other countries.