No products in the cart.
Cerebrolysin, 20 ml 5 pcs
€88.44 €73.70
Description
Notropic drug.
Cerebrolysin contains low molecular weight biologically active neuropeptides, which penetrate through the BBB and directly reach the nerve cells. The drug has an organ-specific multimodal effect on the brain, i.e. provides metabolic regulation, neuroprotection, functional neuromodulation and neurotrophic activity.
Metabolic regulation: the drug Cerebrolysin increases the efficiency of aerobic energy metabolism of the brain, improves intracellular protein synthesis in the developing and aging brain.
Neuroprotection: the drug protects neurons from the damaging effects of lactacidosis, prevents the formation of free radicals, increases survival and prevents neuronal death under conditions of hypoxia and ischemia and reduces the damaging neurotoxic effects of excitatory amino acids (glutamate).
Neurotrophic activity: Cerebrolysin is the only nootropic peptidergic drug with proven neurotrophic activity similar to natural neural growth factors (NGF), but manifested under peripheral administration.
Functional neuromodulation: the drug has a positive effect in cognitive disorders on memory processes.
Indications
Indications
– Alzheimer’s disease;
— dementia syndrome of various origins;
— chronic cerebrovascular insufficiency;
– ischemic stroke;
— traumatic injuries of the brain and spinal cord;
– mental retardation in children;
— disorders associated with attention deficit in children;
– endogenous depression, resistant to antidepressants (as part of complex therapy).
Pharmacological effect
Pharmacological effect
Nootropic drug.
Cerebrolysin contains low molecular weight biologically active neuropeptides that penetrate the BBB and directly enter nerve cells. The drug has an organ-specific multimodal effect on the brain, i.e. provides metabolic regulation, neuroprotection, functional neuromodulation and neurotrophic activity.
Metabolic regulation: Cerebrolysin increases the efficiency of aerobic energy metabolism of the brain, improves intracellular protein synthesis in the developing and aging brain.
Neuroprotection: the drug protects neurons from the damaging effects of lactic acidosis, prevents the formation of free radicals, increases survival and prevents the death of neurons under conditions of hypoxia and ischemia, reduces the damaging neurotoxic effect of excitatory amino acids (glutamate).
Neurotrophic activity: Cerebrolysin is the only nootropic peptidergic drug with proven neurotrophic activity, similar to the action of natural neuronal growth factors (NGF), but manifested under conditions of peripheral administration.
Functional neuromodulation: the drug has a positive effect in cases of cognitive impairment on memory processes.
Special instructions
Special instructions
If injections are performed too quickly, a feeling of heat, sweating, and dizziness may occur. Therefore, the drug should be administered slowly.
The compatibility of the drug has been tested and confirmed (within 24 hours at room temperature and light) with the following standard solutions for infusion: 0.9% sodium chloride solution, Ringer’s solution, 5% dextrose (glucose) solution.
The simultaneous use of Cerebrolysin with vitamins and drugs that improve cardiac circulation is allowed, but these drugs should not be mixed in the same syringe with Cerebrolysin.
Only clear Cerebrolysin solution should be used and only once.
Impact on the ability to drive vehicles and operate machinery
Clinical studies have shown that Cerebrolysin does not affect the ability to drive vehicles and use machinery.
Active ingredient
Active ingredient
Brain peptide complex
Composition
Composition
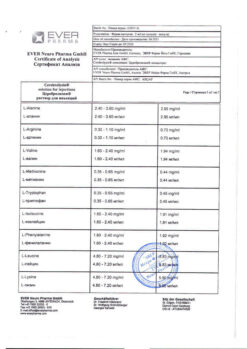
1 ml – Cerebrolysin concentrate (complex of peptides* obtained from pig brain) 215.2 mg
*molecular weight no more than 10,000 daltons.
Pregnancy
Pregnancy
During pregnancy and breastfeeding, Cerebrolysin should be used only after a thorough analysis of the relationship between the positive effect of treatment and the risk associated with it.
The results of experimental studies do not give reason to believe that Cerebrolysin has any teratogenic effect or has a toxic effect on the fetus.
However, similar clinical studies have not been conducted.
Contraindications
Contraindications
– severe renal failure;
– status epilepticus;
– hypersensitivity to the drug.
The drug should be prescribed with caution for allergic diathesis, diseases of an epileptic nature, incl. with generalized epilepsy, due to a possible increase in the frequency of attacks.
Side Effects
Side Effects
The frequency of adverse reactions was determined in accordance with WHO recommendations: very often: (≥1/10); often: (from ≥1/100 to
From the immune system: very rarely – increased individual sensitivity, allergic reactions.
Mental disorders: rarely – the supposed activation effect is accompanied by agitation, manifested by aggressive behavior, confusion, and insomnia.
From the nervous system: rarely – too rapid administration of the drug can lead to dizziness; very rarely – isolated cases of generalized epilepsy and one case of seizures were associated with Cerebrolysin.
From the cardiovascular system: very rarely – too rapid administration of the drug can lead to increased heart rate and arrhythmia.
From the digestive system: very rarely – dyspepsia, diarrhea, constipation, nausea, vomiting; rarely – loss of appetite.
From the skin and subcutaneous tissues: very rarely – skin reactions; rarely – with excessively rapid administration, a feeling of heat, sweating, and itching may occur.
General disorders and disorders at the injection site: very rarely – redness, itching, burning at the injection site, pain in the neck, head and limbs, fever, mild back pain, shortness of breath, chills, collapsed state.
One study reported an association between drug use in rare cases (>1/10,000 to
Since Cerebrolysin is used mainly in elderly patients, the above symptoms of diseases are typical for this age group and often occur without taking the drug.
It should be noted that some undesirable effects (excitement, arterial hypertension, arterial hypotension, lethargy, tremor, depression, apathy, dizziness, headache, shortness of breath, diarrhea, nausea) were identified during clinical studies and occurred equally in both patients receiving Cerebrolysin and patients in the placebo group.
If any of the side effects indicated in the instructions are aggravated or any other side effects not specified in the instructions are noted, the patient should inform the attending physician.
Notification in case of suspected side effects
It is important to report side effects after drug approval to ensure ongoing monitoring of the drug’s risk-benefit ratio. Healthcare professionals are asked to report all cases of adverse reactions observed with the drug through national adverse reaction reporting systems and/or to the company’s representative office.
Interaction
Interaction
Taking into account the pharmacological profile of the drug Cerebrolysin, special attention should be paid to possible additive effects when co-administered with antidepressants or MAO inhibitors. In such cases, it is recommended to reduce the dose of the antidepressant. The use of Cerebrolysin in high doses (30-40 ml) in combination with MAO inhibitors in high doses can cause an increase in blood pressure.
Cerebrolysin and balanced solutions of amino acids should not be mixed in the same solution for infusion.
Cerebrolysin is incompatible with solutions containing lipids and with solutions that change the pH of the medium (5.0-8.0).
Overdose
Overdose
No cases of overdose of Cerebrolysin have been reported.
Recommendations for use
Recommendations for use
The drug is used parenterally. The dose and duration of use depend on the nature and severity of the disease, as well as the age of the patient. A single administration of the drug in a dose of up to 50 ml is possible, but a course of treatment is more preferable.
The recommended course of treatment is daily injections for 10-20 days.
Indication
Dose
Acute conditions (ischemic stroke, traumatic brain injury, complications of neurosurgical operations)
from 10 ml to 50 ml
In the residual period of cerebral stroke and traumatic injury to the brain and spinal cord
from 5 ml to 50 ml
Psychoorganic syndrome and depression
from 5 ml to 30 ml
Alzheimer’s disease, dementia of vascular and combined Alzheimer’s-vascular origin
from 5 ml to 30 ml
In neuropediatric practice
0.1-0.2 ml/kg body weight
To increase the effectiveness of treatment, repeated courses can be carried out as long as the patient’s condition improves as a result of treatment. After the first course, the frequency of injections can be reduced to 2 or 3 times a week.
Cerebrolysin is used parenterally in the form of intramuscular injections (up to 5 ml) and intravenous injections (up to 10 ml). The drug in a dose of 10 ml to 50 ml is recommended to be administered only through slow intravenous infusions after dilution with standard solutions for infusion. The duration of infusions ranges from 15 to 60 minutes.
Storage conditions
Storage conditions
The drug should be stored out of the reach of children, protected from light at a temperature not exceeding 25°C.
Shelf life
Shelf life
The shelf life of the drug in ampoules is 5 years, in vials – 4 years. Do not use after the expiration date stated on the package.
Manufacturer
Manufacturer
EVER Pharma Jena GmbH, Germany
Additional information
| Shelf life | The shelf life of the drug in ampoules is 5 years, in vials – 4 years. Do not use after the expiration date stated on the package. |
|---|---|
| Conditions of storage | The drug should be kept out of reach of children, protected from light at a temperature not exceeding 25°C. |
| Manufacturer | EVER Pharma Jena GmbH, Germany |
| Medication form | solution for injection |
| Brand | EVER Pharma Jena GmbH |
Other forms…
Related products
Buy Cerebrolysin, 20 ml 5 pcs with delivery to USA, UK, Europe and over 120 other countries.