No products in the cart.
Ceftriaxone, 1 g
€1.56 €1.42
Out of stock
(E-mail when Stock is available)
Description
Ceftriaxone – cephalosporin antibiotic of III generation for parenteral use, has bactericidal action, inhibits cell membrane synthesis, in vitro suppresses the growth of most Gram-positive and Gram-negative microorganisms. Ceftriaxone is resistant to beta-lactamase enzymes (both penicillinase and cephalosporinase produced by most Gram-positive and Gram-negative bacteria). In vitro and in clinical practice ceftriaxone is generally effective against the following microorganisms:
Gram-positive:
Staphylococcus aureus, Staphylococcus epidermidis, Streptococcus pneumoniae, Streptococcus A (Str.pyogenes), Streptococcus V (Str. agalactiae), Streptococcus viridans, Streptococcus bovis.
Note: Staphylococcus spp. resistant to methicillin is also resistant to cephalosporins, including ceftriaxone. Most strains of enterococci (e.g., Streptococcus faecalis) are also resistant to ceftriaxone.
Gram-negative:
Aeromonas spp., Alcaligenes spp., Branhamella catarrhalis, Citrobacter spp, Enterobacter spp. (some strains are resistant), Escherichia coli, Haemophilus ducreyi, Haemophilus influenzae, Haemophilus parainfluenzae, Klebsiella spp. (including Kl. pneumoniae), Moraxella spp, Morganella morganii, Neisseria gonorrhoeae, Neisseria meningitidis, Plesiomonas shigelloides, Proteus mirabilis, Proteus vulgaris, Providencia spp., Pseudomonas aeruginosa (some strains are resistant), Salmonella spp. (including S. typhi), Serratia spp, Vibrio spp. (including V. cholerae), Yersinia spp. (including Y. enterocolitica)
Note: Many strains of the listed microorganisms, which in the presence of other antibiotics, such as penicillins, first generation cephalosporins and aminoglycosides multiply steadily, are sensitive to ceftriaxone. Treponema pallidum is sensitive to ceftriaxone both in vitro and in animal experiments. According to clinical data in primary and secondary syphilis good effectiveness of ceftriaxone is noted.
Anaerobic pathogens:
Bacteroides spp. (including some strains of B. fragilis), Clostridium spp. (including CI. difficile), Fusobacterium spp. (except F. mostiferum. F. varium), Peptococcus spp., Peptostreptococcus spp.
Note: Some strains of many Bacteroides spp. (for example, B. fragilis), producing beta-lactamase, are resistant to ceftriaxone. To determine the sensitivity of microorganisms it is necessary to use disks containing ceftriaxone, because it has been shown that in vitro certain strains of pathogens may be resistant to classical cephalosporins.
Pharmacokinetics:
When administered parenterally, ceftriaxone penetrates well into tissues and body fluids. In healthy adult subjects, ceftriaxone is characterized by a long, about 8 hours, half-life. The areas under the concentration-time curve in blood serum when administered intravenously and intramuscularly coincide. This means that bioavailability of ceftriaxone with intramuscular administration is 100%. When administered intravenously, ceftriaxone rapidly diffuses into the interstitial fluid, where it retains its bactericidal effect against pathogens sensitive to it for 24 hours.
The half-life in healthy adult subjects is about 8 hours. In newborns under 8 days and in older adults over 75 years of age, the average half-life is about twice as long. In adults, 50-60% of ceftriaxone is excreted unchanged in the urine and 40-50% is also excreted unchanged in the bile. Under the influence of intestinal flora ceftriaxone is converted into an inactive metabolite. In infants about 70% of the administered dose is excreted by the kidneys. In adults with renal insufficiency or liver pathology pharmacokinetics of ceftriaxone is almost unchanged, the elimination half-period is lengthened slightly. If renal function is impaired, excretion with bile increases, and if there is hepatic pathology, renal excretion of ceftriaxone increases.
Ceftriaxone reversibly binds to albumin and this binding is inversely proportional to the concentration: for example, at a drug concentration in serum of less than 100 mg/l the binding of ceftriaxone to proteins is 95%, and at a concentration of 300 mg/l – only 85%. Due to the lower content of albumin in interstitial fluid, the concentration of ceftriaxone in it is higher than in blood serum.
Perfusion into the cerebrospinal fluid: In infants and children with cerebral membrane inflammation, ceftriaxone penetrates into the cerebrospinal fluid, with an average of 17% of the drug concentration in serum diffusing into the cerebrospinal fluid in bacterial meningitis, which is about 4 times greater than in aseptic meningitis.
24 hours after intravenous administration of ceftriaxone at a dose of 50-100 mg/kg body weight, the concentration in the cerebrospinal fluid exceeds 1.4 mg/L. In adult meningitis patients, 2-25 hours after administration of ceftriaxone at a dose of 50 mg/kg body weight, the concentration of ceftriaxone was many times greater than the minimum suppressive dose necessary to suppress the pathogens that most frequently cause meningitis.
Indications
Indications
Infections caused by pathogens sensitive to ceftriaxone: sepsis, meningitis, abdominal infections (peritonitis, inflammatory diseases of the gastrointestinal tract, biliary tract), infections of bones, joints, connective tissue, skin, infections in patients with reduced immune system function, kidney and urinary tract infections, respiratory tract infections, especially pneumonia, as well as infections of the ear, nose and throat, genital infections, including gonorrhea.
Prevention of infections in the postoperative period.
Pharmacological effect
Pharmacological effect
Ceftriaxone is a third-generation cephalosporin antibiotic for parenteral use, has a bactericidal effect, inhibits cell membrane synthesis, and in vitro inhibits the growth of most Gram-positive and Gram-negative microorganisms. Ceftriaxone is resistant to beta-lactamase enzymes (both penicillinase and cephalosporinase, produced by most Gram-positive and Gram-negative bacteria). In vitro and in clinical practice, ceftriaxone is usually effective against the following microorganisms:
Gram-positive:
Staphylococcus aureus, Staphylococcus epidermidis, Streptococcus pneumoniae, Streptococcus A (Str. pyogenes), Streptococcus V (Str. agalactiae), Streptococcus viridans, Streptococcus bovis.
Note: Staphylococcus spp., resistant to methicillin, is also resistant to cephalosporins, including ceftriaxone. Most strains of enterococci (eg Streptococcus faecalis) are also resistant to ceftriaxone.
Gram-negative:
Aeromonas spp., Alcaligenes spp., Branhamella catarrhalis, Citrobacter spp., Enterobacter spp. (some strains are resistant), Escherichia coli, Haemophilus ducreyi, Haemophilus influenzae, Haemophilus parainfluenzae, Klebsiella spp. (including Kl. pneumoniae), Moraxella spp., Morganella morganii, Neisseria gonorrhoeae, Neisseria meningitidis, Plesiomonas shigelloides, Proteus mirabilis, Proteus vulgaris, Providencia spp., Pseudomonas aeruginosa (some strains are resistant), Salmonella spp. (including S. typhi), Serratia spp. (including S. marcescens), Shigella spp., Vibrio spp. (including V. cholerae), Yersinia spp. (including Y. enterocolitica)
Note: Many strains of the listed microorganisms, which multiply steadily in the presence of other antibiotics, for example, penicillins, first-generation cephalosporins and aminoglycosides, are sensitive to ceftriaxone. Treponema pallidum is sensitive to ceftriaxone both in vitro and in animal experiments. According to clinical data, ceftriaxone has good efficacy in primary and secondary syphilis.
Anaerobic pathogens:
Bacteroides spp. (including some strains of B. fragilis), Clostridium spp. (including CI. difficile), Fusobacterium spp. (except F. mostiferum. F. varium), Peptococcus spp., Peptostreptococcus spp.
Note: Some strains of many Bacteroides spp. (eg B. fragilis) that produce beta-lactamase are resistant to ceftriaxone. To determine the sensitivity of microorganisms, it is necessary to use disks containing ceftriaxone, since it has been shown that in vitro certain strains of pathogens can be resistant to classical cephalosporins.
Pharmacokinetics:
When administered parenterally, ceftriaxone penetrates well into body tissues and fluids. In healthy adult subjects, ceftriaxone has a long half-life of about 8 hours. The areas under the concentration-time curve in blood serum after intravenous and intramuscular administration are the same. This means that the bioavailability of ceftriaxone when administered intramuscularly is 100%. When administered intravenously, ceftriaxone quickly diffuses into the interstitial fluid, where it retains its bactericidal effect against pathogens sensitive to it for 24 hours.
The half-life in healthy adult subjects is approximately 8 hours. In neonates up to 8 days and in elderly people over 75 years of age, the average half-life is approximately twice as long. In adults, 50-60% of ceftriaxone is excreted unchanged in the urine, and 40-50% is also excreted unchanged in the bile. Under the influence of intestinal flora, ceftriaxone is converted into an inactive metabolite. In newborns, approximately 70% of the administered dose is excreted by the kidneys. In case of renal failure or liver pathology in adults, the pharmacokinetics of ceftriaxone remains almost unchanged, the elimination half-life is slightly extended. If renal function is impaired, excretion into bile increases, and if liver pathology occurs, then excretion of ceftriaxone by the kidneys increases.
Ceftriaxone binds reversibly to albumin and this binding is inversely proportional to the concentration: for example, at a drug concentration in the blood serum of less than 100 mg/l, the protein binding of ceftriaxone is 95%, and at a concentration of 300 mg/l it is only 85%. Due to the lower albumin content in the interstitial fluid, the concentration of ceftriaxone in it is higher than in the blood serum.
Penetration into the cerebrospinal fluid: In newborns and children with inflammation of the meninges, ceftriaxone penetrates into the cerebrospinal fluid, while in the case of bacterial meningitis, an average of 17% of the drug concentration in the blood serum diffuses into the cerebrospinal fluid, which is approximately 4 times more than in aseptic meningitis.
24 hours after intravenous administration of ceftriaxone at a dose of 50-100 mg/kg body weight, the concentration in the cerebrospinal fluid exceeds 1.4 mg/l. In adult patients with meningitis, 2-25 hours after administration of ceftriaxone at a dose of 50 mg/kg body weight, the concentration of ceftriaxone many times exceeded the minimum inhibitory dose necessary to suppress the pathogens that most often cause meningitis.
Special instructions
Special instructions
Despite a detailed history taking, which is the rule for other cephalosporin antibiotics, the possibility of developing anaphylactic shock cannot be ruled out, which requires immediate treatment – first, adrenaline is administered intravenously, then glucocorticoids.
Sometimes an ultrasound examination of the gallbladder reveals the presence of a shadow, indicating sedimentation. This symptom disappears after completion or temporary cessation of ceftriaxone therapy. Even in the presence of pain, such cases do not require surgical intervention; conservative treatment is sufficient.
In vitro studies have shown that, like other cephalosporin antibiotics, ceftriaxone is able to displace bilirubin bound to serum albumin. Therefore, in newborns with hyperbilirubinemia and, especially, in premature newborns, the use of ceftriaxone requires even greater caution. Since the drug passes into breast milk, breastfeeding should not be continued during treatment with ceftriaxone.
With long-term use, periodic monitoring of the blood count is necessary. Ceftriaxone is used only in hospital settings.
Active ingredient
Active ingredient
Ceftriaxone
Composition
Composition
One bottle contains 1.0 g of Ceftriaxone sodium salt.
Contraindications
Contraindications
Hypersensitivity to cephalosporins and penicillins. First trimester of pregnancy.
Side Effects
Side Effects
Systemic side effects:
from the gastrointestinal tract (about 2% of patients): diarrhea, nausea, vomiting, stomatitis and glossitis.
Changes in the blood picture (about 2% of patients) in the form of eosinophilia, leukopenia, granulocytopenia, hemolytic anemia, thrombocytopenia.
Skin reactions (about 1% of patients) in the form of exanthema, allergic dermatitis, urticaria, edema, erythema multiforme.
Other rare side effects: headaches, dizziness, increased liver enzymes, gallbladder congestion, oliguria, increased serum creatinine, mycoses in the genital area, chills, anaphylaxis or anaphylactic reactions. Pseudomembranous enterocolitis and blood clotting disorders are extremely rare.
Local side effects:
After intravenous administration, phlebitis was observed in some cases.
This phenomenon can be prevented by slow (over 2-4 minutes) administration of the drug. The described side effects usually disappear after stopping therapy.
Overdose
Overdose
Excessively high plasma concentrations of ceftriaxone cannot be lowered by hemodialysis or peritoneal dialysis.
Symptomatic measures are recommended to treat cases of overdose.
Shelf life
Shelf life
See packaging.
Manufacturer
Manufacturer
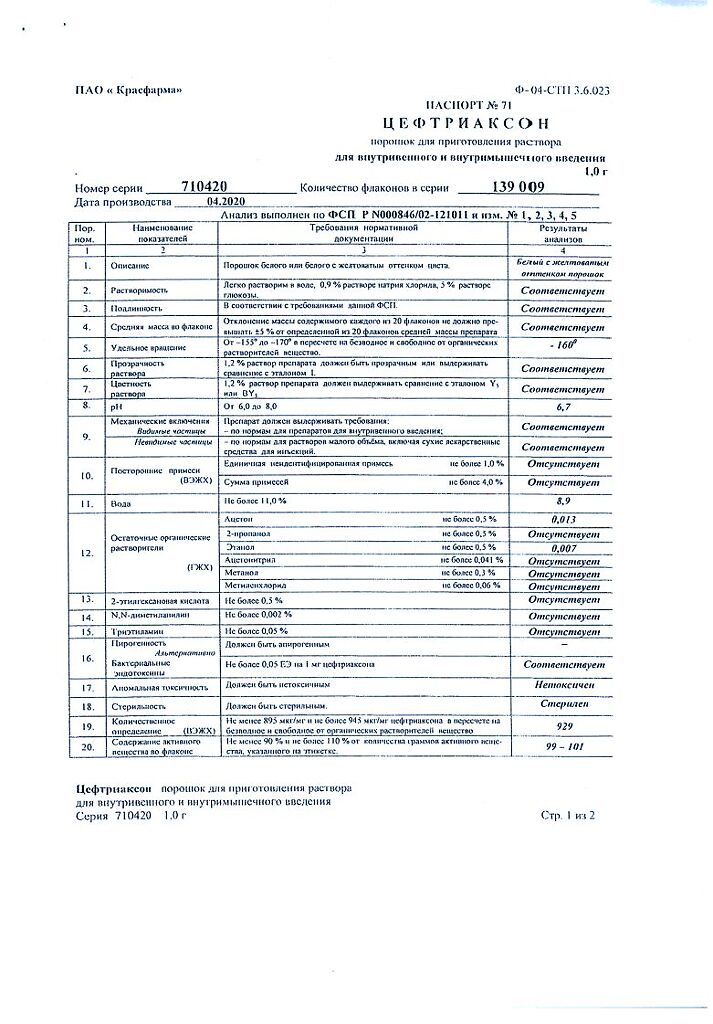
Kraspharma PJSC, Russia
Additional information
| Shelf life | See on the package. |
|---|---|
| Manufacturer | Kraspharma PJSC, Russia |
| Medication form | Powder for preparation of solution |
| Brand | Kraspharma PJSC |
Other forms…
Related products
Buy Ceftriaxone, 1 g with delivery to USA, UK, Europe and over 120 other countries.