No products in the cart.
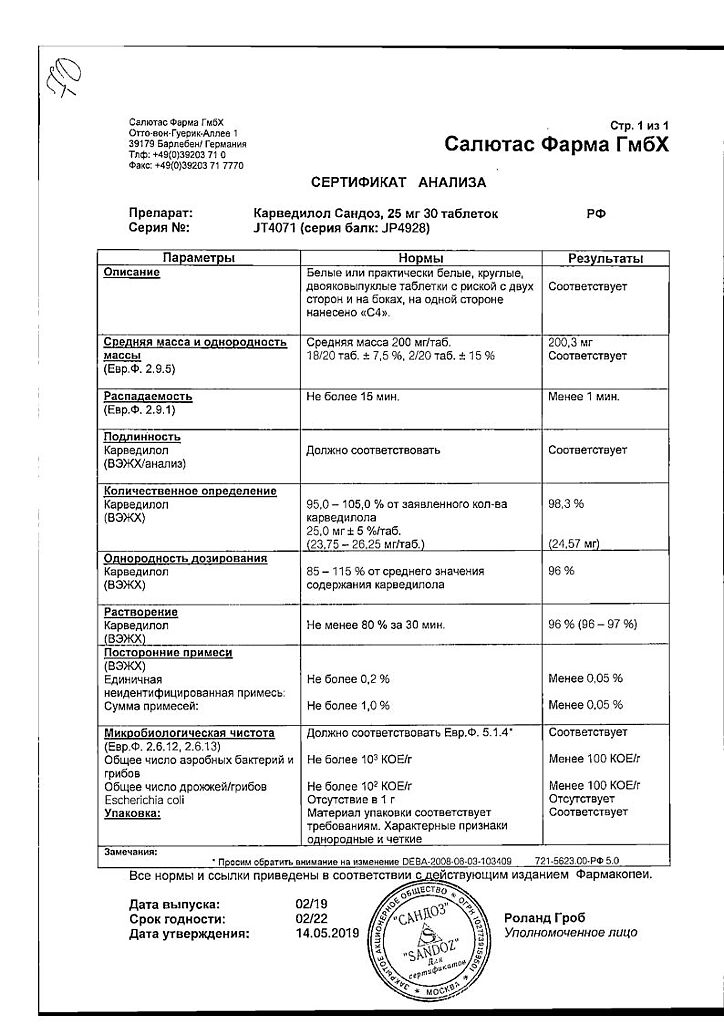
Carvedilol Sandoz, tablets 25 mg 30 pcs
€9.64 €8.04
Description
Carvedilol is alpha- and beta-adrenoblocking, hypotensive, antioxidant, antianginal.
Pharmacodynamics
It has a combined non-selective beta-blocking, alpha1-blocking and antioxidant effect. The vasodilatory effect is mainly due to blockade of alpha1 receptors. Due to vasodilatation, it decreases RPS. It has no intrinsic sympathomimetic activity and, like propranololol, has a membrane stabilizing effect.
Powerful antioxidant, eliminates free oxygen radicals. The combination of the vasodilator action and the beta-adrenoblocking properties of carvedilol leads to the fact that in patients with arterial hypertension, a decrease in BP is not accompanied by a simultaneous increase in RPS, which is observed when taking beta-adrenoblockers. HR decreases insignificantly, renal blood flow and renal function are preserved. Because peripheral blood flow is preserved, limb cooling is very rare, unlike in patients treated with beta-adrenoblockers.
The antihypertensive effect develops quickly – 2-3 hours after a single dose and lasts for 24 hours. With prolonged treatment maximum effect is observed after 3-4 weeks. In patients with CHD, carvedilol has anti-ischemic and antianginal effects. It reduces pre- and afterload on the heart. It has no pronounced effect on lipid metabolism and plasma content of potassium, sodium and magnesium ions. In patients with left ventricular dysfunction and/or circulatory insufficiency, the alpha1-adrenoblocking effect of carvedilol leads to dilation of arterial and, to a lesser extent, venous vessels; carvedilol has favorable effects on hemodynamic parameters: increases left ventricular ejection function and reduces its size.
Carvedilol has beneficial effects on cardiac hemodynamics and left ventricular ejection fraction in both dilated cardiomyopathy and ischemic heart failure. In heart failure, end-systolic and end-diastolic volume as well as peripheral and pulmonary vascular resistance decrease. Ejection fraction and cardiac index do not change in normal heart function.
It is established that when additionally prescribed against the background of taking cardiac glycosides, ACE inhibitors and diuretics, carvedilol decreases mortality rate, slows disease progression and improves general condition of the patient regardless of disease severity. The effect of carvedilol is more pronounced in patients with tachycardia (HR greater than 82 bpm) and low ejection fraction (less than 23%).
The HDL/LDL cholesterol ratio does not change during treatment with carvedilol.
Pharmacokinetics
Absorption of carvedilol is fast and high. Tmax is 1 h after drug administration. Plasma concentration is proportional to the dose taken. The bioavailability is about 30%. Simultaneous intake of food slows down absorption of the drug, but does not affect the value of bioavailability.
The binding to plasma proteins is about 98-99%. Vd is about 2 l/kg and increases (by 80%) in case of liver dysfunction (due to reduced effect of first passage through the liver). Metabolized mainly in the liver due to intensive coupling with glucuronic acid. By demethylation and hydroxylation of the phenyl ring three active metabolites with pronounced antioxidant and adrenoblocking properties are formed. T1/2 of carvedilol is 6-10 h, plasma clearance is about 590 ml/min. It is excreted mainly with the bile and a small part is excreted through the kidneys.
It penetrates the placental barrier and is excreted with breast milk.
Carvedilol is practically not excreted by hemodialysis. In cirrhosis, the bioavailability of carvedilol is 4 times higher and the Cmax in plasma is 5 times higher than normal. Carvedilolol is mainly excreted through the gastrointestinal tract, so no cumulation of the drug is observed in renal dysfunction.
It should be noted that in elderly patients the plasma concentration of Carvedilol is 50% higher than in younger patients.
Indications
Indications
arterial hypertension in the form of mono- or combination therapy (in combination with thiazide diuretics);
stable angina;
chronic heart failure in combination with diuretics, digoxin or ACE inhibitors.
Pharmacological effect
Pharmacological effect
Carvedilol – alpha and beta adrenergic blocking, hypotensive, antioxidant, antianginal.
Pharmacodynamics
It has a combined non-selective beta-blocking, alpha1-blocking and antioxidant effect. The vasodilating effect is associated mainly with the blockade of alpha1 receptors. Thanks to vasodilation, it reduces peripheral vascular resistance. It does not have internal sympathomimetic activity and, like propranolol, has a membrane-stabilizing effect.
Powerful antioxidant, eliminates free oxygen radicals. The combination of the vasodilatory effect and beta-blocking properties of carvedilol leads to the fact that in patients with arterial hypertension, a decrease in blood pressure is not accompanied by a simultaneous increase in peripheral vascular resistance, which is observed when taking beta-blockers. Heart rate decreases slightly, renal blood flow and renal function are preserved. Since peripheral blood flow is preserved, coldness of the extremities is very rare, in contrast to patients treated with beta-blockers.
The antihypertensive effect develops quickly – 2-3 hours after a single dose and continues for 24 hours. With prolonged treatment, the maximum effect is observed after 3-4 weeks. In patients with ischemic heart disease, carvedilol has anti-ischemic and antianginal effects. Reduces pre- and afterload on the heart. Does not have a pronounced effect on lipid metabolism and the content of potassium, sodium and magnesium ions in plasma. In patients with impaired left ventricular function and/or circulatory insufficiency, the alpha1-adrenergic blocking effect of carvedilol leads to dilation of arterial and, to a lesser extent, venous vessels; Carvedilol has a beneficial effect on hemodynamic parameters: it increases the ejection function of the left ventricle and reduces its size.
Carvedilol has a beneficial effect on cardiac hemodynamics and left ventricular ejection fraction in both dilated cardiomyopathy and ischemic heart failure. In heart failure, end-systolic and end-diastolic volume decreases, as well as peripheral and pulmonary vascular resistance. Ejection fraction and cardiac index do not change with normal cardiac function.
It has been established that when additionally prescribed against the background of cardiac glycosides, ACE inhibitors and diuretics, carvedilol reduces the mortality rate, slows the progression of the disease and improves the general condition of the patient, regardless of the severity of the disease. The effect of carvedilol is more pronounced in patients with tachycardia (heart rate more than 82 beats/min) and low ejection fraction (less than 23%).
During treatment with carvedilol, the HDL/LDL cholesterol ratio does not change.
Pharmacokinetics
Absorption of carvedilol is rapid and high. Tmax is 1 hour after taking the drug. Plasma concentration is proportional to the dose taken. The bioavailability value is about 30%. Simultaneous food intake slows down the absorption of the drug, but does not affect the amount of bioavailability.
Plasma protein binding is about 98–99%. Vd is about 2 l/kg and increases (by 80%) in case of liver dysfunction (due to a decrease in the first-pass effect through the liver). Metabolized primarily in the liver due to intensive combination with glucuronic acid. By demethylation and hydroxylation of the phenyl ring, three active metabolites with pronounced antioxidant and adrenergic blocking properties are formed. T1/2 of carvedilol is 6–10 hours, plasma clearance is about 590 ml/min. It is excreted mainly with bile and a small part through the kidneys.
Penetrates the placental barrier and is excreted in breast milk.
Carvedilol is practically not excreted during hemodialysis. In liver cirrhosis, the bioavailability of carvedilol is 4 times higher, and Cmax in blood plasma is 5 times higher than normal. Carvedilol is excreted mainly through the gastrointestinal tract, therefore, in case of impaired renal function, drug accumulation is not observed.
It should be taken into account that in elderly patients the concentration of carvedilol in the blood plasma is 50% higher than in young patients.
Special instructions
Special instructions
If the heart rate decreases to 55 beats/min, the drug should be discontinued. In individuals with allergies or undergoing desensitization, taking carvedilol may increase allergy sensitivity.
Contact lens wearers should be warned that the drug reduces tear production. When completing a course of treatment with Carvedilol Sandoz, simultaneously with clonidine, first gradually reduce the dosage of clonidine and then first discontinue clonidine and then carvedilol.
If it is necessary to use Carvedilol Sandoz during lactation, breastfeeding should be stopped. During the treatment period, it is necessary to avoid alcohol consumption.
If circulatory failure progresses during treatment, it is recommended to increase the dose of diuretics; in case of renal failure, adjust the dose taking into account indicators of the functional state of the kidneys.
If surgery using general anesthesia is necessary, the anesthesiologist should be warned about previous therapy with carvedilol. It should be borne in mind that the drug can mask the symptoms of thyrotoxicosis and hypoglycemia, therefore it is recommended to regularly monitor blood glucose levels and, if necessary, adjust doses.
Active ingredient
Active ingredient
Carvedilol
Composition
Composition
Active ingredient:
carvedilol 25 mg;
Excipients:
lactose monohydrate;
MCC;
crospovidone;
povidone K30;
colloidal silicon dioxide;
magnesium stearate
Pregnancy
Pregnancy
The drug is contraindicated during pregnancy.
If it is necessary to use the drug Carvedilol Sandoz during lactation, breastfeeding should be discontinued.
Contraindications
Contraindications
hypersensitivity to carvedilol and other components of the drug;
chronic heart failure in the stage of decompensation;
sick sinus syndrome;
AV block II-III stage, with the exception of patients with an artificial pacemaker;
severe bradycardia (heart rate less than 50 beats/min);
cardiogenic shock, collapse;
bronchial asthma;
severe liver dysfunction;
metabolic acidosis;
pregnancy;
lactation period;
age under 18 years (efficacy and safety have not been established);
joint intravenous administration of verapamil, diltiazem or other antiarrhythmic drugs (especially class I antiarrhythmic drugs).
With caution and under control:
diabetes mellitus;
hypoglycemia;
thyrotoxicosis;
pheochromocytoma (only stabilized by the administration of alpha-blockers);
occlusive diseases of peripheral vessels;
AV block of the first degree;
COPD
Prinzmetal’s angina;
psoriasis;
renal dysfunction;
depression;
myasthenia gravis;
treatment with alpha-blockers and alpha-agonists;
simultaneous use with digitalis preparations, diuretics and/or MAO inhibitors;
old age.
Side Effects
Side Effects
At recommended doses, Carvedilol is well tolerated, but side effects are possible in some cases.
From the digestive system: nausea, dry mouth, abdominal pain, diarrhea or constipation, vomiting, increased activity of liver transaminases.
From the nervous system: headache, dizziness, feeling of fatigue, loss of consciousness, muscle weakness (usually at the beginning of treatment), sleep disturbance, depression, paresthesia.
From the senses: decreased tear production.
From the genitourinary system: impaired renal function, edema, impaired urination.
From the hematopoietic organs: leukopenia, thrombocytopenia.
Allergic reactions: urticaria, itching, rashes, appearance and/or exacerbation of psoriasis, sneezing, nasal congestion, bronchospasm, shortness of breath (in predisposed patients), very rarely – anaphylactoid reaction.
From the cardiovascular system: bradycardia, orthostatic hypotension, angina pectoris, AV block, progression of circulatory failure (cold extremities), progression of heart failure, exacerbation of intermittent claudication syndrome, Raynaud’s syndrome.
Endocrine disorders: weight gain.
Other: pain in the limbs, flu-like symptoms.
As with the use of other drugs that block alpha-adrenergic receptors, latent diabetes mellitus may appear or its symptoms may intensify.
Interaction
Interaction
Catecholamine depleting agents (reserpine, MAO inhibitors) can cause severe bradycardia and hypotension. Concomitant administration of ACE inhibitors, thiazide diuretics, and vasodilators prescribed simultaneously with carvedilol can lead to a sharp drop in blood pressure.
Enhances the effect of insulin and sulfonylurea derivatives (while masking or weakening the severity of symptoms of hypoglycemia, reducing the breakdown of liver glycogen to glucose).
When coadministered with insulin or oral hypoglycemic drugs, blood glucose levels should be monitored. Inhibitors of the CYP2D6 isoenzyme (quinidine, fluoxetine, propafenone) may increase the concentration of the R(+)-enantiomer of carvedilol.
Combined use with antiarrhythmic drugs (especially class I) and BMCC (verapamil, diltiazem) can provoke severe arterial hypotension and heart failure. Intravenous administration of these drugs together with carvedilol is contraindicated.
Increases the concentration of digoxin, which requires monitoring its concentration, because simultaneous administration with cardiac glycosides can lead to AV blockade.
General anesthetics enhance negative inotropic and hypotensive effects. Phenobarbital and rifampicin accelerate metabolism and reduce plasma concentrations of carvedilol. Inhibitors of microsomal oxidation (cimetidine), diuretics and ACE inhibitors increase the concentration and enhance the hypotensive effect of carvedilol. Carvedilol delays the metabolism of cyclosporine.
Overdose
Overdose
Symptoms: pronounced decrease in blood pressure (sBP 80 mmHg and below), severe bradycardia (less than 50 beats/min), impaired respiratory function (bronchospasm, etc.), chronic circulatory failure, cardiogenic shock, cardiac arrest.
Treatment: during the first two hours, induce vomiting and rinse the stomach. An overdose requires intensive treatment, monitoring of vital functions is necessary. The patient should be in a position with his legs elevated, i.e. in the Trendelenburg position. Beta-blocking antidotes include orciprenaline or isoprenaline 0.5–1 mg IV and/or glucagon 1–5 mg (maximum dose 10 mg). Severe hypotension is treated with parenteral fluids and repeated administration of epinephrine 5–10 mg (or IV infusion at a rate of 5 mcg/min).
For excessive bradycardia, atropine is prescribed intravenously at a dose of 0.5–2 mg. To maintain cardiac activity: glucagon is administered intravenously quickly (within 30 seconds), followed by a continuous infusion at a rate of 2–5 mg/hour. If the peripheral vasodilatory effect predominates (warm extremities, in addition to significant arterial hypotension), it is necessary to prescribe norepinephrine 5-10 mcg as an intravenous infusion – 5 mcg/min.
To relieve bronchospasm, beta-agonists (in the form of an aerosol or IV) or aminophylline IV are prescribed. If convulsions develop, slow administration of diazepam or clonazepam is recommended. In severe cases of intoxication, when symptoms of shock dominate, treatment should continue until the patient’s condition stabilizes, taking into account the T1/2 of carvedilol, which is 6–10 hours.
Storage conditions
Storage conditions
At a temperature not exceeding 25 °C.
Shelf life
Shelf life
3 years.
Manufacturer
Manufacturer
Salutas Pharma GmbH, Germany
Additional information
| Shelf life | 3 years. |
|---|---|
| Conditions of storage | At a temperature not exceeding 25 °C. |
| Manufacturer | Salutas Pharma GmbH, Germany |
| Medication form | pills |
| Brand | Salutas Pharma GmbH |
Other forms…
Related products
Buy Carvedilol Sandoz, tablets 25 mg 30 pcs with delivery to USA, UK, Europe and over 120 other countries.