No products in the cart.
Cardiomagnil, 150 mg+30.39 mg 30 pcs
€7.16 €6.26
Description
Antiplatelet.
Acetylsalicylic acid irreversibly inhibits the enzyme cyclooxygenase and selectively reduces the synthesis of thromboxane A2, which leads to reduced platelet aggregation and decreased blood clotting.
Magnesium hydroxide protects the mucous membranes of the gastrointestinal tract, which reduces the risk of side effects.
Indications
Indications
prevention of thrombosis and embolism;
prevention of myocardial infarction (including unstable angina);
prevention of ischemic cerebrovascular accidents;
transient transient cerebral ischemic attacks;
postoperative period after surgical interventions on the heart and blood vessels (including after coronary artery bypass grafting and percutaneous transluminal coronary angioplasty).
Pharmacological effect
Pharmacological effect
Antiplatelet agent.
Acetylsalicylic acid irreversibly inhibits the enzyme cyclooxygenase and selectively reduces the synthesis of thromboxane A2, which leads to a decrease in platelet aggregation and a decrease in blood clotting.
Magnesium hydroxide protects the mucous membranes of the gastrointestinal tract, which reduces the risk of side effects.
Special instructions
Special instructions
The drug is prescribed with caution to patients with a history of gastric ulcer.
The patient should be warned about the need to consult a doctor if any adverse events occur.
Active ingredient
Active ingredient
Acetylsalicylic acid, [Magnesium hydroxide]
Composition
Composition
White, oval, film-coated tablets with a score line on one side.
Active ingredients
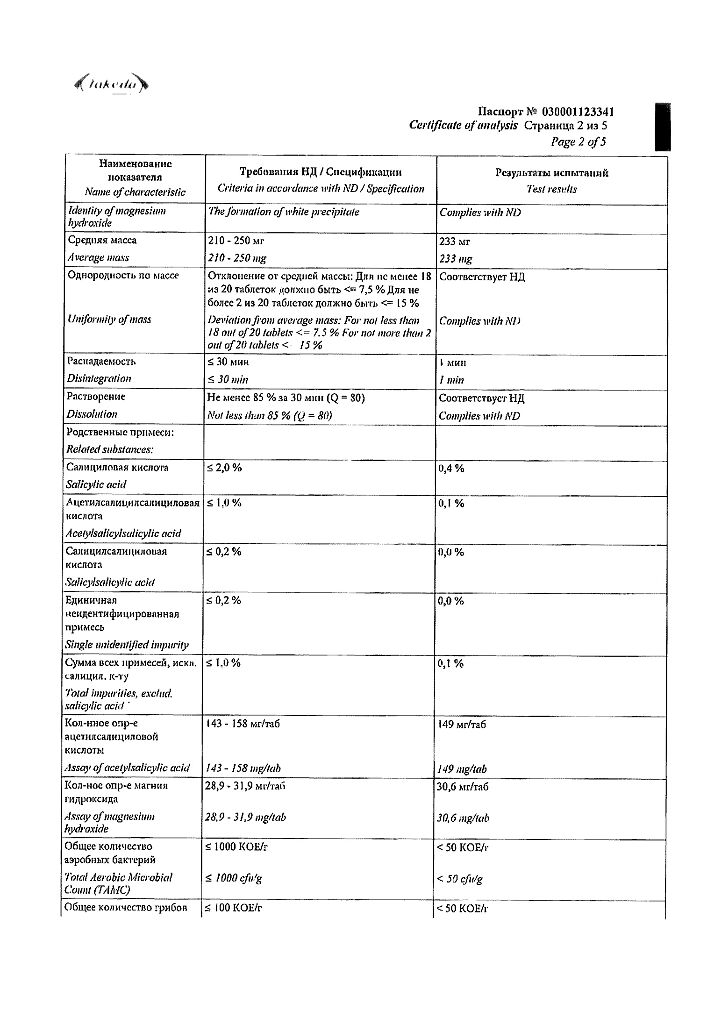
Acetylsalicylic acid 150 mg in 1 tablet.
Magnesium hydroxide 30.39 mg in 1 tablet.
Excipients
Corn starch.
Microcrystalline cellulose.
Magnesium stearate.
Potato starch.
Hypromellose (methylhydroxypropylcellulose 15).
Macrogol (propylene glycol).
Talc.
Contraindications
Contraindications
history of bleeding in the gastrointestinal tract;
history of cerebrovascular accidents of the hemorrhagic type;
thrombocytopenia;
hemophilia;
hemorrhagic diathesis;
tendency to bleed;
hypoprothrombinemia;
bronchial asthma;
deficiency of glucose-6-phosphate dehydrogenase;
renal failure;
I and III trimester of pregnancy;
lactation (breastfeeding);
children and adolescents up to 18 years of age;
hypersensitivity to acetylsalicylic acid or salicylates.
Pregnancy and lactation The drug is contraindicated for use in the first and third trimesters of pregnancy and during lactation (breastfeeding).
Interaction
Interaction
When Cardiomagnyl is used in high doses simultaneously with oral anticoagulants, the effect of the latter may be enhanced.
When used together, Cardiomagnyl (in high doses) weakens the uricosuric effect of probenecid.
With simultaneous use, Cardiomagnyl enhances the effect of chlorpropamide, antiplatelet agents, fibrinolytic agents and weakens the effect of spironolactone.
When taken simultaneously, antacids and cholestyramine reduce the absorption of acetylsalicylic acid.
Overdose
Overdose
For adults, a dose of the drug corresponding to 150 mg of acetylsalicylic acid per 1 kg of body weight is dangerous.
Symptoms: tinnitus, decreased hearing acuity, dizziness, increased sweating, anxiety, abdominal pain, nausea, vomiting (possibly with blood), increased breathing, increased bleeding time; rarely – cardiac dysfunction; in isolated cases – pulmonary edema.
Treatment: if necessary, carry out symptomatic therapy.
Storage conditions
Storage conditions
In a place protected from light and moisture at a temperature of 15-30 °C.
Keep out of the reach of children.
Manufacturer
Manufacturer
Takeda Pharmaceuticals LLC, Russia
Additional information
| Conditions of storage | Store in a place protected from light and moisture at 15-30 °C. Keep out of reach of children. |
|---|---|
| Manufacturer | Takeda Pharmaceuticals LLC, Russia |
| Medication form | pills |
| Brand | Takeda Pharmaceuticals LLC |
Other forms…
Related products
Buy Cardiomagnil, 150 mg+30.39 mg 30 pcs with delivery to USA, UK, Europe and over 120 other countries.