No products in the cart.
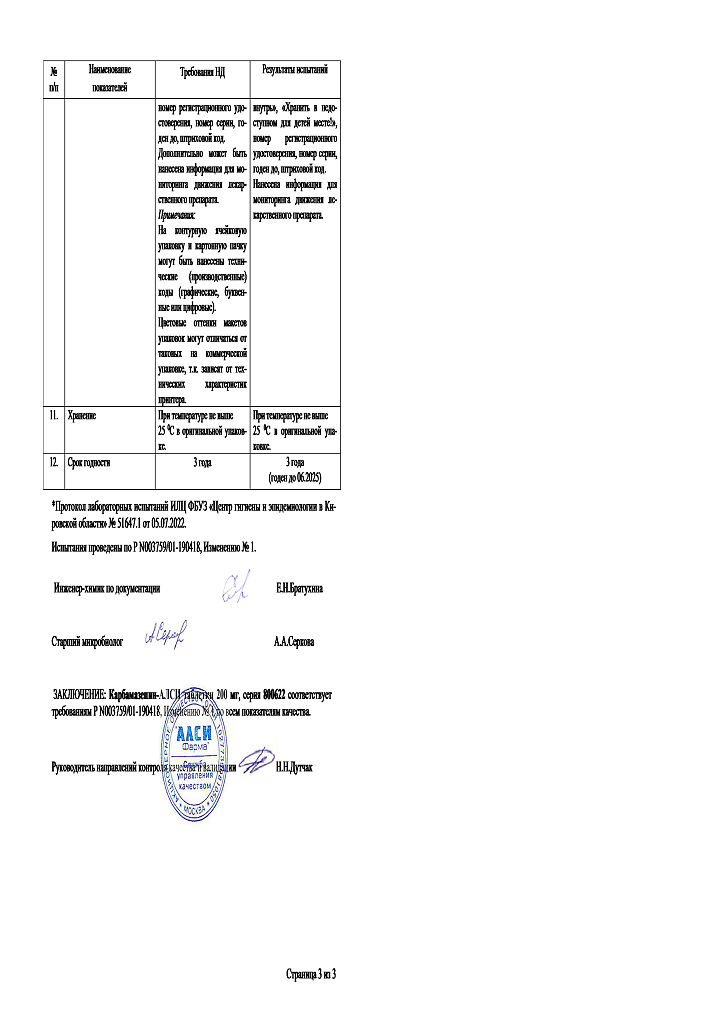
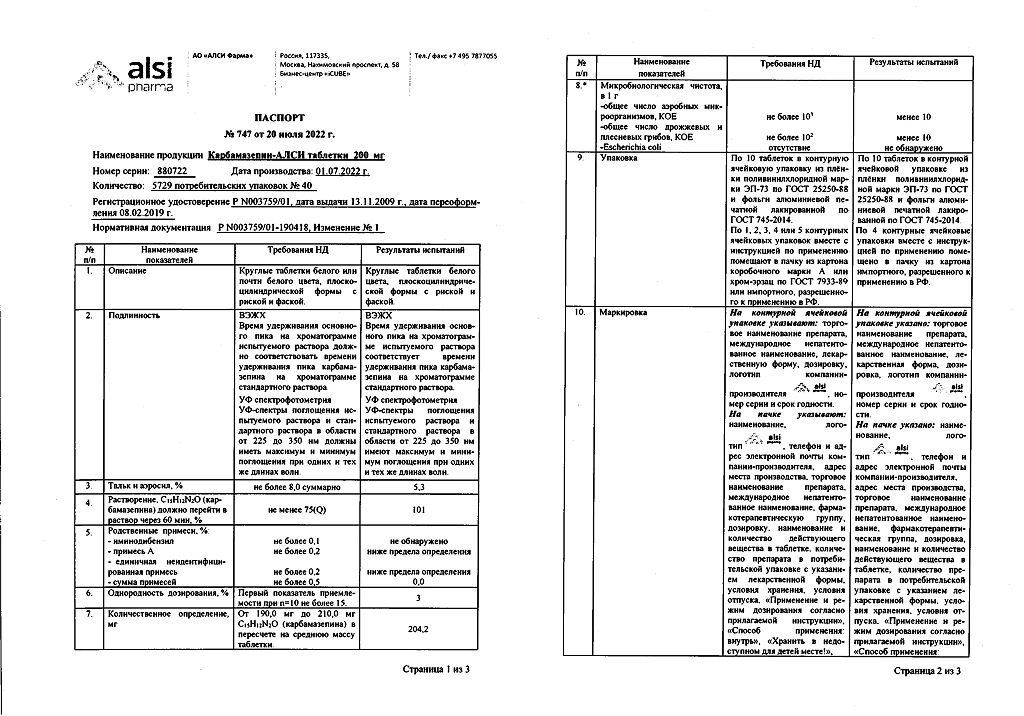
Carbamazepine-ALSI, tablets 200 mg 40 pcs
€4.45 €3.71
Out of stock
(E-mail when Stock is available)
Description
Carbamazepine is a dibenzoazepine derivative and has antiepileptic, neurotropic, psychotropic and antidiuretic effects.
As an antiepileptic, it stabilizes the membranes of overexcited neurons, suppresses serial discharges of neurons and reduces synaptic transmission of excitatory impulses.
This action is presumably achieved through blockade of sodium channels, which prevents the reappearance of sodium-dependent action potentials in depolarized neurons. Reduces the release of the neurotransmitter glutamate.
The psychotropic effects of carbamazepine appear to be due to inhibition of dopamine and noradrenaline metabolism.
It reduces seizure frequency, anxiety, depression, irritability and aggressiveness in patients with epilepsy. Effects on cognitive function in patients with epilepsy are variable.
Prevent the appearance of paroxysmal pain in neuralgia. In alcohol withdrawal syndrome increases the threshold of seizure readiness, reduces increased nervous excitability, tremor, gait disturbances.
It is used for the treatment of affective disorders as an antipsychotic and normothetic agent. In non-sugar diabetes it reduces diuresis and feeling of thirst.
Indications
Indications
Epilepsy (monotherapy or as part of complex therapy):
– complex and simple partial seizures (with or without loss of consciousness) with or without secondary generalization;
– generalized tonic-clonic seizures. Mixed forms of epileptic seizures.
Acute manic states and maintenance therapy of bipolar affective disorders to prevent exacerbations or reduce the severity of clinical manifestations.
In complex therapy of alcohol withdrawal syndrome.
Trigeminal neuralgia (idiopathic, in multiple sclerosis), idiopathic glossopharyngeal neuralgia.
Pain syndrome in diabetic neuropathy.
Polyuria and polydipsia of neurohormonal nature in diabetes insipidus.
Pharmacological effect
Pharmacological effect
Carbamazepine is a derivative of dibenzoazepine and has antiepileptic, neurotropic, psychotropic and antidiuretic effects.
As an antiepileptic drug, it stabilizes the membranes of overexcited neurons, suppresses serial discharges of neurons and reduces the synaptic transmission of excitatory impulses.
This effect is achieved presumably by blocking sodium channels, thereby preventing the re-occurrence of sodium-dependent action potentials in depolarized neurons. Reduces the release of the neurotransmitter glutamate.
The psychotropic effect of carbamazepine is apparently due to inhibition of the metabolism of dopamine and norepinephrine.
Reduces the frequency of attacks, anxiety, depression, irritability and aggressiveness in patients with epilepsy. The effect on cognitive function in patients with epilepsy is variable.
Prevents the appearance of paroxysmal pain due to neuralgia. In case of alcohol withdrawal syndrome, it increases the threshold of convulsive readiness, reduces increased nervous excitability, tremor, and gait disturbances.
Used to treat affective disorders as an antipsychotic and mood stabilizer. In diabetes insipidus, it reduces diuresis and thirst.
Special instructions
Special instructions
Before starting treatment, as well as periodically during treatment, clinical blood tests (including counting the number of platelets, reticulocytes, and iron concentration in the blood serum), general urine tests and determination of the level of urea in the blood should be performed. The drug has weak anticholinergic activity. Therefore, when using the drug in patients with increased intraocular pressure, constant monitoring of this indicator is necessary.
It is recommended to periodically determine the concentration of carbamazepine in the blood plasma in cases of increased frequency of epileptic seizures, in the treatment of children, in pregnant women, in the case of its use as part of complex therapy, or in the development of severe side effects.
There are isolated reports of disturbances in male fertility and/or disturbances in spermatogenesis. However, the causal relationship of these disorders with taking the drug has not yet been proven.
Cross-hypersensitivity reactions may occur between carbamazepine and phenytoin or oxcarbazepine.
During the treatment period, it is necessary to refrain from engaging in potentially hazardous activities that require increased concentration and speed of psychomotor reactions.
During therapy, it is necessary to stop drinking alcohol, since carbamazepine enhances the inhibitory effect of alcohol on the central nervous system.
Active ingredient
Active ingredient
Carbamazepine
Composition
Composition
One tablet contains:
active substance:
carbamazepine 200 mg;
excipients:
potato starch – 80.5 mg,
colloidal silicon dioxide (Aerosil) – 16.4 mg,
talc – 3.1 mg,
magnesium stearate – 3.1 mg,
povidone (polyvinylpyrrolidone) – 14.4 mg,
polysorbate (Tween-80) – 2.5 mg.
Contraindications
Contraindications
Hypersensitivity to any of the components of the drug or chemically similar drugs (tricyclic antidepressants),
atrioventricular block,
history of suppression of bone marrow hematopoiesis,
hepatic porphyrias,
simultaneous use of monoamine oxidase inhibitors (hereinafter referred to as MAO inhibitors) and for 2 weeks after their discontinuation,
breastfeeding period.
Use with caution when: low levels of leukocytes or platelets; mixed forms of epileptic seizures, including absence seizures; in old age; with heart, liver or kidney failure; increased intraocular pressure; dilution hyponatremia; hypothyroidism; prostatic hyperplasia; pregnancy (increased risk of developing intrauterine development disorders, including malformations).
Side Effects
Side Effects
Nervous system disorders: dizziness, ataxia, drowsiness, fatigue, headache, diplopia, accommodation disturbances, tremor, muscular dystonia, tics, nystagmus, orofacial dyskinesia, oculomotor disorders, dysarthria, choreoathetoid disorders, peripheral neuropathy, paresthesia, paresis, taste disturbances, neuroleptic malignant syndrome.
Mental disorders: hallucinations (visual or auditory), depression, anorexia, anxiety, aggressive behavior, agitation, disorientation, increased psychosis.
From the skin and its appendages: allergic dermatitis, urticaria, exfoliative dermatitis, erythroderma, systemic lupus erythematosus, itching, Stevens-Johnson syndrome, toxic epidermal necrolysis, photosensitivity reactions, erythema multiforme and erythema nodosum, skin pigmentation disorders, purpura, acne, sweating, hair loss. Rare cases of hirsutism have been reported, but the causal relationship of this complication to the drug remains unclear.
From the hematopoietic system: leukopenia, thrombocytopenia, eosinophilia, leukocytosis, lymphadenopathy, folic acid deficiency, agranulocytosis, aplastic anemia, pancytopenia, anemia, true erythrocyte aplasia, megaloblastic anemia, variegated porphyria, porphyria cutanea tarda, acute intermittent porphyria, reticulocytosis, hemolytic anemia.
From the hepatobiliary system: increased levels of gamma-glutamine transferase, alkaline phosphatase, transaminases; hepatitis (cholestatic, parenchymal (hepatocellular) or mixed type), jaundice, granulomatous hepatitis, liver failure.
From the gastrointestinal tract: nausea, vomiting, dry mouth, diarrhea, constipation, abdominal pain, glossitis, stomatitis, pancreatitis.
Hypersensitivity reactions: multiorgan delayed-type hypersensitivity with fever, skin rash, vasculitis, lymphadenopathy, arthralgia, leukopenia, eosinophilia, hepatosplenomegaly and altered liver function tests (these manifestations occur in various combinations). Other organs may also be involved (lungs, kidneys, pancreas, myocardium, colon). Aseptic meningitis with myoclonus and eosinophilia; anaphylactic reaction, angioedema.
From the cardiovascular system: intracardiac conduction disorders; decrease or increase in blood pressure, bradycardia, arrhythmia, atrioventricular block with fainting, collapse, congestive heart failure, worsening of coronary heart disease, thrombophlebitis, thromboembolism.
From the endocrine system and metabolism: edema, fluid retention, weight gain, hyponatremia and a decrease in plasma osmolarity due to an effect similar to the action of antidiuretic hormone, which in rare cases leads to water intoxication (dilution hyponatremia), accompanied by lethargy, vomiting, headache, disorientation and neurological disorders; increased prolactin levels, accompanied or not accompanied by galactorrhea, gynecomastia; decreased concentrations of triiodothyronine and thyroxine, increased concentrations of thyroid-stimulating hormone, which is usually not accompanied by clinical manifestations; disorders of bone tissue metabolism (decrease in the concentration of calcium and 25-hydroxycolecalciferol in the blood plasma), which leads to osteomalacia; increased concentrations of cholesterol, including high-density lipoprotein cholesterol and triglycerides.
From the genitourinary system: interstitial nephritis, renal failure, albuminuria, hematuria, oliguria, azotemia, frequent urination, urinary retention, sexual function disorders, spermatogenesis disorders.
From the senses: disturbances in taste, clouding of the lens, increased intraocular pressure, conjunctivitis; hearing disorders.
From the musculoskeletal system: arthralgia, muscle pain, muscle weakness, cramps.
From the respiratory system: hypersensitivity reactions characterized by fever, shortness of breath, pneumonitis or pneumonia.
Changes in laboratory results: hypogammaglobulinemia.
Interaction
Interaction
Concomitant use with inhibitors of the CYP3A4 isoenzyme may lead to increased plasma concentrations of carbamazepine. Concomitant use of inducers of the CYP3A4 isoenzyme may lead to an acceleration of the metabolism of carbamazepine and a possible decrease in its plasma concentration. Withdrawal of concomitantly taken inducers of the CYP3A4 isoenzyme may reduce the rate of biotransformation of carbamazepine and lead to an increase in the level of carbamazepine in the blood plasma. When used simultaneously with drugs metabolized by the CYP3A4 isoenzyme, induction of metabolism and a decrease in their concentration in plasma is possible.
Drugs that may increase plasma concentrations of carbamazepine or carbamazepine-10,11-epoxide:
dextropropoxyphene, ibuprofen, danazol, macrolide antibiotics (eg, erythromycin, troleandomycin, josamycin, clarithromycin), fluoxetine, fluvoxamine, nefazodone, paroxetine, trazodone, viloxazine, stiripentol, vigabatrin, azoles (eg, itraconazole, ketoconazole, fluconazole, voriconazole), loratadine, terfenadine, loxapine, olanzapine, quetiapine, isoniazid, viral protease inhibitors for the treatment of HIV infection (for example, ritonavir), acetazolamide, verapamil, diltiazem, omeprazole, oxybutynin, dantrolene, ticlopidine, nicotinamide (in adults, only in high doses), possibly cimetidine, desipramine, primidone, valproic acid.
Drugs that may reduce plasma concentrations of carbamazepine:
felbamate, methsuximide, oxcarbazepine, phenobarbital, fensuximide, phenytoin, fosphenytoin, primidone, progabide, theophylline, aminophylline, isotretinoin, rifampicin, cisplatin, doxorubicin; herbal preparations containing St. John’s wort and, although data are conflicting, possibly also clonazepam, valproic acid or valpromide.
The effect of carbamazepine on plasma concentrations of drugs used as concomitant therapy:
Carbamazepine may reduce plasma concentrations or reduce or even completely eliminate the effects of the following drugs: methadone, paracetamol, antipyrine, tramadol, doxycycline, oral anticoagulants (warfarin, phenprocoumon, dicumarol, acenocoumarol), bupropion, citalopram, trazodone, tricyclic antidepressants (imipramine, amitriptyline, nortriptyline, clomipramine), clobazam, clonazepam, ethosuximide, felbamate, lamotrigine, oxcarbazepine, primidone, tiagabine, topiramate, valproic acid, zonisamide, itraconazole, praziquantel, imatinib, clozapine, haloperidol, bromperidol, olanzapine, quetiapine, risperidone, ziprasidone, used in the treatment of HIV infection (indinavir, ritonavir, saquinavir), alprazolam; midazolam, theophylline, calcium channel blockers of the dihydropyridine group (for example, felodipine), digoxin, oral contraceptives (selection of alternative methods of contraception is necessary), glucocorticosteroids (for example, prednisolone, dexamethasone); cyclosporine, everolimus, levothyroxine sodium, estrogens and/or progesterone. There are reports that while taking carbamazepine, the level of phenytoin in the blood plasma can either increase or decrease, and the level of mephenytoin can increase (in rare cases).
Combinations to consider:
Increased hepatotoxicity caused by isoniazid is possible when used simultaneously with carbamazepine.
In the case of co-administration with levetiracetam, the toxic effect of carbamazepine may be enhanced.
The combined use of carbamazepine and lithium or metoclopramide, as well as carbamazepine and antipsychotic drugs (haloperidol, thioridazine) can lead to an increase in the frequency of adverse neurological reactions (in the case of the latter combination, even at therapeutic concentrations of active substances in the blood plasma).
The simultaneous use of carbamazepine with some diuretics (hydrochlorothiazide, furosemide) can lead to hyponatremia, accompanied by clinical manifestations.
Carbamazepine may antagonize the action of non-depolarizing muscle relaxants (eg, pancuronium bromide). If such a combination of drugs is used, it may be necessary to increase the dose of these muscle relaxants; Patients should be closely monitored as the effects of muscle relaxants may cease more quickly than expected.
Coadministration with grapefruit juice may increase plasma levels of carbamazepine.
Overdose
Overdose
Symptoms
– central nervous system: depression of the functions of the central nervous system up to coma, disorientation, drowsiness, agitation, hallucinations, a feeling of “fog” before the eyes, dysarthria, nystagmus, ataxia, dyskinesia, hyperreflexia alternating with hyporeflexia, convulsions, psychomotor disorders, myoclonus, hypothermia, mydriasis;
– respiratory system: respiratory depression, pulmonary edema;
– cardiovascular system: tachycardia, decrease or increase in blood pressure, cardiac conduction disturbances with widening of the QRS complex, cardiac arrest;
– digestive system: vomiting, delayed evacuation of food from the stomach, decreased colon motility;
– urinary system: urinary retention, oliguria or anuria, fluid retention, dilution hyponatremia;
– laboratory parameters: metabolic acidosis, hyperglycemia, increased muscle fraction of creatine phosphokinase, hyponatremia.
Treatment: there is no specific antidote. Gastric lavage, administration of activated charcoal (late evacuation of gastric contents can lead to delayed absorption for 2-3 days and reappearance of intoxication symptoms), hospitalization, symptomatic therapy. Forced diuresis, hemodialysis and peritoneal dialysis are ineffective (dialysis is indicated for a combination of severe poisoning and renal failure). It is recommended to carry out hemosorption on carbon sorbents.
Recommendations for use
Recommendations for use
Orally, regardless of food intake, with a small amount of liquid.
Epilepsy: Whenever possible, carbamazepine should be prescribed as monotherapy. Treatment begins with a small daily dose, which is subsequently slowly increased until the optimal effect is achieved. The addition of carbamazepine to existing antiepileptic therapy should be carried out gradually.
For adults, the initial dose is 100-200 mg 1-2 times a day. Then the dose is slowly increased to 400 mg 2-3 times a day. The maximum daily dose is 2000 mg.
For children under 5 years of age, the initial dose is 20-60 mg per day, increasing by 20-60 mg every two days. In children over 5 years of age, the initial dose is 100 mg/day, followed by an increase of 100 mg/week. The maintenance dose for children is 10-20 mg/kg body weight per day in 2-3 doses. To ensure accurate dosing, liquid oral dosage forms of carbamazepine must be used in children under 5 years of age.
Neuralgia of the trigeminal or glossopharyngeal nerves: initial dose 200-400 mg/day, then the dose is gradually increased by no more than 200 mg per day until the pain stops (on average, up to 600-800 mg), then reduced to the minimum effective dose. When treating elderly patients, the initial dose is 100 mg 2 times a day.
Alcohol withdrawal syndrome: average dose – 200 mg 3 times a day. In severe cases, in the first days, the dose can be increased to 400 mg 3 times a day. At the beginning of treatment for severe withdrawal symptoms, it is prescribed in combination with detoxification therapy, sedatives and hypnotics.
Polyuria and polydipsia in diabetes insipidus: the average dose for adults is 200 mg 2 – 3 times a day. In children, the dose is selected taking into account body weight and age.
Pain syndrome in diabetic neuropathy: 200 mg 2 – 4 times a day.
Acute manic states and maintenance therapy of bipolar affective disorders: daily dose 400-1600 mg (average daily dose 200-600 mg) in 2-3 doses per day. In acute cases, the dose is increased quickly enough. During maintenance therapy, dose increases should be gradual and small.
Functional features
Functional features
Absorption is slow, but quite complete (food intake does not significantly affect the speed and degree of absorption). After a single dose, the maximum concentration in the blood plasma is reached after 12 hours. Equilibrium concentrations of the drug in plasma are achieved after 1-2 weeks.
Among patients, there are significant individual differences in the value of equilibrium concentrations in the therapeutic range. The connection with blood plasma proteins is 70–80%. The concentration of unchanged carbamazepine in the cerebrospinal fluid and saliva is proportional to the amount of active substance not bound to proteins (20–30%). The concentration in breast milk is 25–60% of that in plasma. Penetrates through the placental barrier.
Metabolized in the liver, mainly along the epoxide pathway with the formation of metabolites: active – carbamazepine-10,11-epoxide and low-active – 9-hydroxy-methyl-10 carbamoylacridan. The main isoenzyme that ensures the biotransformation of carbamazepine into carbamazepine-10,11-epoxide is the cytochrome P450 3A4 isoenzyme. The content of carbamazepine-10,11-epoxide is about 30% of the plasma level of carbamazepine. The biotransformation of carbamazepine-10,11-epoxide into carbamazepine-10,11-transdiol occurs with the help of the microsomal enzyme epoxide hydrolase.
Another pathway of carbamazepine metabolism is the formation of various monohydroxylated derivatives, as well as N-glucuronides. The half-life after a single dose averages about 36 hours (ranges from 25 to 65 hours), after repeated doses – 16-24 hours. In patients receiving additional antiepileptic drugs that induce liver enzymes, an average of 9-10 hours.
It is excreted primarily as inactive metabolites in urine (approximately 70%) and feces (approximately 30%). About 2% is excreted in the urine as unchanged carbamazepine and 1% as carbamazepine-10,11-epoxide.
In children, due to the faster elimination of carbamazepine, higher doses of the drug per kilogram of body weight may be required compared to adults.
There is no evidence that the pharmacokinetics of carbamazepine changes in elderly patients. There are no data on the pharmacokinetics of carbamazepine in patients with impaired renal or hepatic function.
Storage conditions
Storage conditions
In a dry place, protected from light, at a temperature not exceeding 25°C.
Keep out of the reach of children.
Shelf life
Shelf life
3 years. Do not use after expiration date.
Manufacturer
Manufacturer
ALSI Pharma, Russia
Additional information
| Shelf life | 3 years. Do not use after the expiration date. |
|---|---|
| Conditions of storage | In a dry, light-protected place at a temperature not exceeding 25°C. Store out of the reach of children. |
| Manufacturer | ALSI Pharma, Russia |
| Medication form | pills |
| Brand | ALSI Pharma |
Other forms…
Related products
Buy Carbamazepine-ALSI, tablets 200 mg 40 pcs with delivery to USA, UK, Europe and over 120 other countries.