No products in the cart.
Calmirex, 2.5mg/ml+100 mg/ml 10 pcs.
€24.38 €21.13
Description
Tolperisone hydrochloride is a myorelaxant of central action. It has membrane stabilizing, local anesthetic effect, inhibits conduction of nerve impulses in primary afferent fibers and motor neurons, which leads to blocking of spinal mono- and polysynaptic reflexes.
Probably mediates the blocking of mediator release by inhibition of calcium ion entry into synapses. Inhibits the conduction of excitation along the reticulospinal pathway in the brainstem.
Independent on the CNS it increases peripheral blood flow. The weak antispasmodic and antiadrenergic effects of tolperizone may play a role in the development of this effect.
Lidocaine hydrochloride has local anesthetic effect and has no systemic action when dosed according to instructions.
Indications
Indications
– hypertonicity and spasm of the transverse striated musculature arising from organic diseases of the CNS (including lesions of the pyramidal pathways, multiple sclerosis, stroke, myelopathy, encephalomyelitis), the musculoskeletal system (including spondylosis, spondyloarthrosis, cervical and lumbar syndromes of large joints.
– rehabilitation treatment after orthopedic and trauma surgeries.
In complex therapy:
– in obliterating vascular diseases (obliterating atherosclerosis, diabetic angiopathy, obliterating thrombangiitis, Raynaud’s disease, diffuse scleroderma);
– in diseases arising from vascular innervation disorder (acrocyanosis, intermittent angioneurotic dysbasia).
Active ingredient
Active ingredient
How to take, the dosage
How to take, the dosage
Interaction
Interaction
There are no data on drug interactions that limit the use of Calmirex®.
While tolperizone has an effect on the CNS, the drug does not cause sedation, so it can be used in combination with sedatives, hypnotics and drugs containing ethanol.
It does not increase the effect of ethanol on the CNS.
It potentiates the effects of NSAIDs; therefore, with concomitant use, it may be necessary to reduce the dose of the latter.
Special Instructions
Special Instructions
Impact on driving and operating machinery
Perform caution when driving motor vehicles and engaging in other potentially dangerous activities that require increased concentration and quick psychomotor reactions.
Contraindications
Contraindications
– severe myasthenia gravis;
– pregnancy;
– lactation period;
– childhood and adolescence under 18 years of age;
– hypersensitivity to the components of the drug, including lidocaine.
– hypersensitivity to the ingredients of the drug, including lidocaine.
The drug should be used with caution in cases of renal and hepatic impairment (dose adjustment is not required).
Side effects
Side effects
Frequency of side effects: very frequently (â¥1/10), frequently (â¥1/100 to < 1/10), infrequently (â¥1/1000 to < 1/100), rarely (â¥1/10 000 to < 1/1000), very rarely (< 1/10 000), frequency unknown (cannot be estimated from available data).
Hematopoietic system disorders: very rare – anemia, lymphadenopathy.
The immune system: rare – hypersensitivity reactions, anaphylactic reactions, very rare – anaphylactic shock.
Metabolism and nutrition: infrequent – anorexia; very rare – polydipsia.
Mental disorders: infrequent – insomnia, sleep disorders, rarely – loss of activity, depression.
Nervous system disorders: infrequent – headache, dizziness, somnolence; rare – attention deficit syndrome, tremor, seizures, loss of sensitivity, sensory disorders, lethargy.
An organ of vision: rarely – disorders of visual perception.
Hearing organ and labyrinth disorders: rarely – tinnitus, vertigo.
Cardiovascular system: infrequent – arterial hypotension; rare – angina, tachycardia, palpitations; very rare – bradycardia, hot flashes.
Respiratory system: rarely – shortness of breath, nasal bleeding, rapid breathing.
The digestive system: infrequent – gastrointestinal discomfort, diarrhea, dry mouth, dyspepsia, nausea; rarely – epigastric pain, constipation, flatulence, vomiting, liver function disorders.
Skin and subcutaneous tissue disorders: rare – allergic dermatitis, sweating, skin itching, urticaria, skin rash.
Muscular system: infrequent – muscle weakness, myalgia, pain in the extremities, rarely – discomfort in the extremities, very rarely – osteopenia.
The urinary system: rarely – enuresis, proteinuria.
General disorders and disorders at the injection site: often – redness of the injection site; infrequent – asthenia (weakness), malaise, fatigue; rarely – feeling of intoxication, feeling of heat, irritability, thirst; very rarely – feeling of discomfort in the chest.
Laboratory and instrumental data: rarely – decreased BP, increased bilirubin concentration, impaired liver function tests, decreased platelet count, increased white blood cell count; very rarely – increased creatinine level.
Overdose
Overdose
Symptoms: ataxia, tonic and clonic convulsions, dyspnea, respiratory arrest.
Treatment: symptomatic and supportive therapy is recommended. There is no specific antidote.
Pregnancy use
Pregnancy use
Additional information
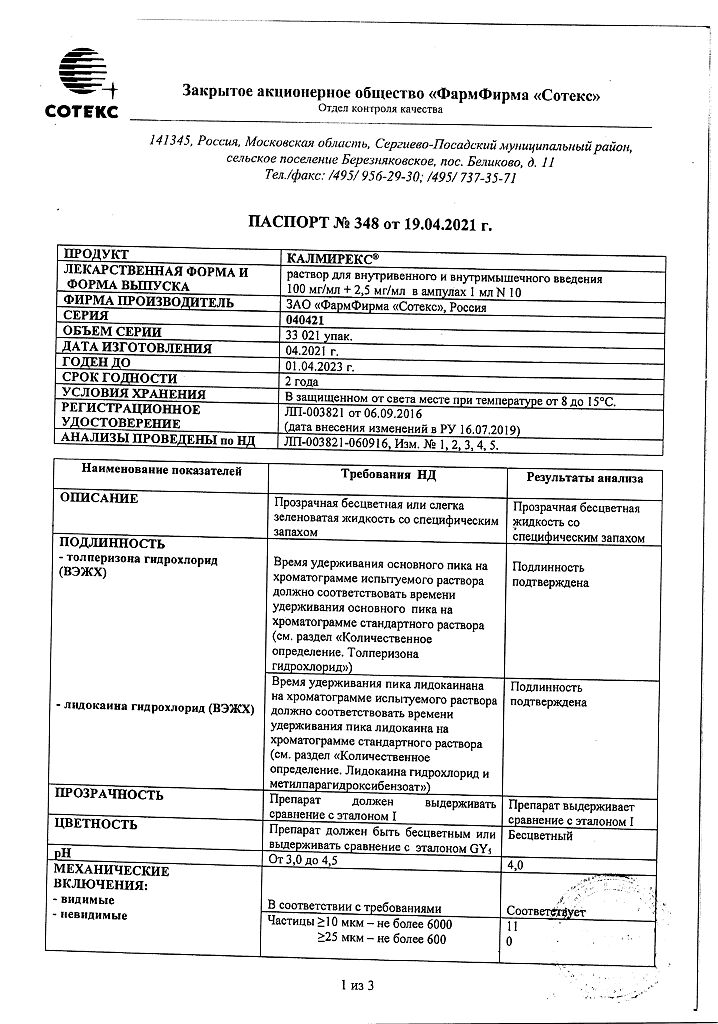
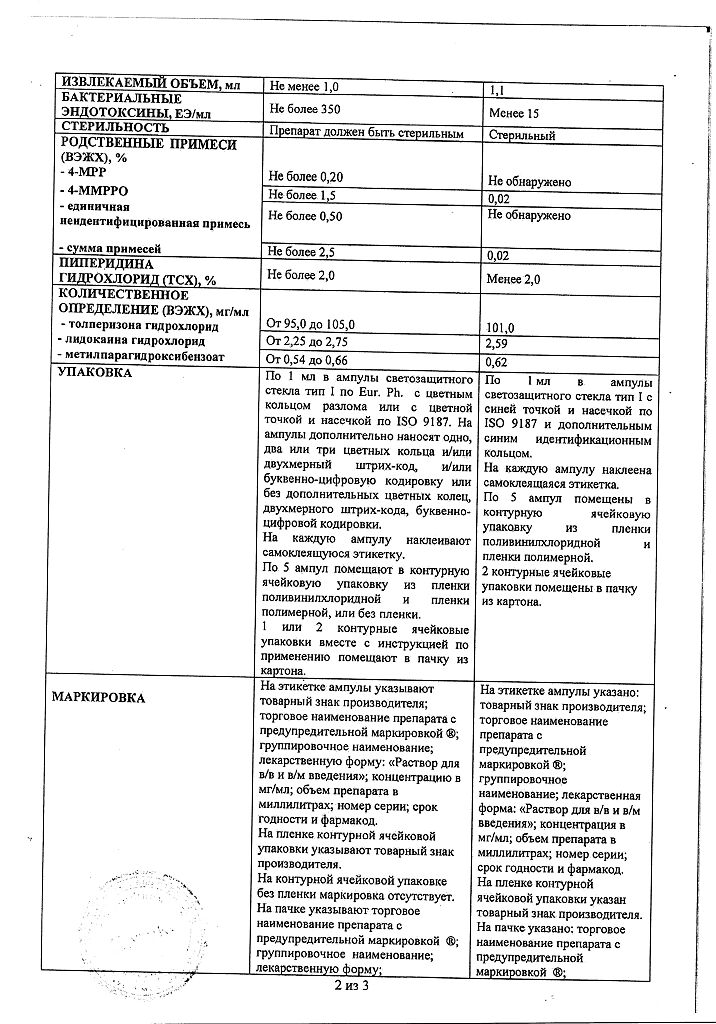
| Shelf life | 2 years. |
|---|---|
| Conditions of storage | The drug should be kept out of reach of children, protected from light at 8 ° C to 15 ° C. |
| Manufacturer | PharmFirm Sotex, Russia |
| Medication form | solution |
| Brand | PharmFirm Sotex |
Other forms…
Related products
Buy Calmirex, 2.5mg/ml+100 mg/ml 10 pcs. with delivery to USA, UK, Europe and over 120 other countries.