No products in the cart.
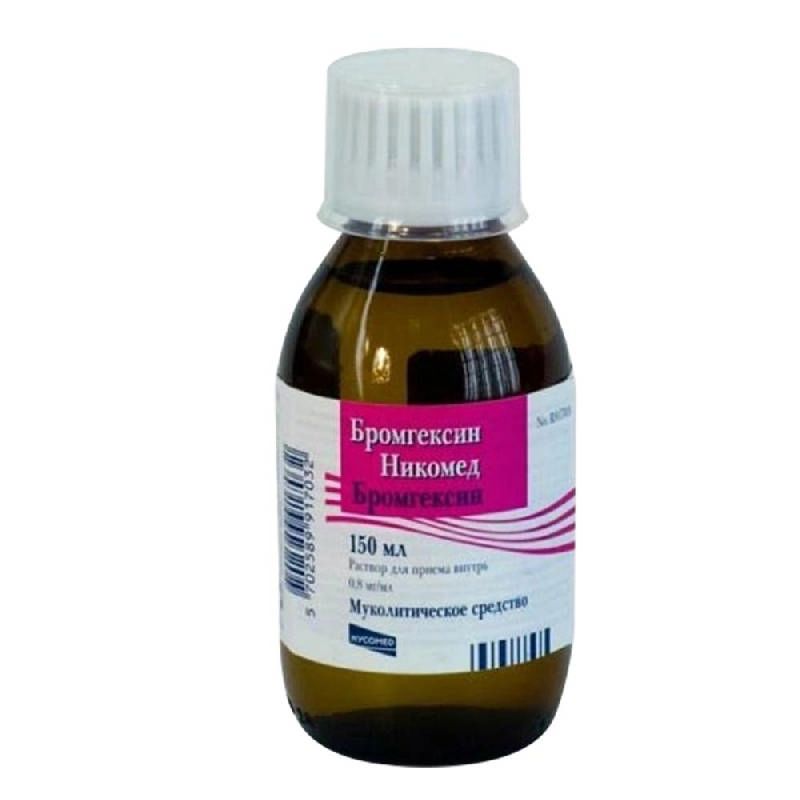
Bromhexin Nicomed, 0.8 mg/ml 150 ml
€8.52 €7.46
Description
Mucolytic agent with expectorant action. It reduces the viscosity of bronchial secretion due to depolarization of acidic polysaccharides contained in it and stimulation of secretory cells of the bronchial mucosa that produce secretion containing neutral polysaccharides. It is believed that bromhexin promotes the formation of surfactant.
Pharmacokinetics
Bromhexine is rapidly absorbed from the GI tract and undergoes intensive metabolism during “first passage” through the liver. Bioavailability is about 20%. In healthy patients Cmax in plasma is determined after 1 hour.
Widely distributed in body tissues. About 85-90% is excreted with urine mainly in the form of metabolites. The metabolite of bromhexin is ambroxol.
The binding of bromhexine to plasma proteins is high. T1/2 in the terminal phase is about 12 hours.
Bromhexin penetrates through the HEB. In small amounts it penetrates through the placental barrier.
Only small amounts are excreted in the urine with a T1/2 of 6.5 h.
The clearance of bromhexine or its metabolites may be decreased in patients with severe hepatic and renal impairment.
Indications
Indications
Diseases of the respiratory tract accompanied by the formation of difficult-to-discharge viscous secretions: tracheobronchitis, chronic bronchitis with a broncho-obstructive component, bronchial asthma, cystic fibrosis, chronic pneumonia.
Pharmacological effect
Pharmacological effect
Mucolytic agent with expectorant action. Reduces the viscosity of bronchial secretions by depolarizing the acidic polysaccharides it contains and stimulating the secretory cells of the bronchial mucosa, which produce secretions containing neutral polysaccharides. It is believed that bromhexine promotes the formation of surfactant.
Pharmacokinetics
Bromhexine is rapidly absorbed from the gastrointestinal tract and undergoes intensive metabolism during the “first pass” through the liver. Bioavailability is about 20%. In healthy patients, Cmax in plasma is determined after 1 hour.
Widely distributed in body tissues. About 85-90% is excreted in the urine, mainly in the form of metabolites. Ambroxol is a metabolite of bromhexine.
The binding of bromhexine to plasma proteins is high. T1/2 in the terminal phase is about 12 hours.
Bromhexine penetrates the BBB. In small quantities it penetrates the placental barrier.
Only small amounts are excreted in urine with a T1/2 of 6.5 hours.
The clearance of bromhexine or its metabolites may be reduced in patients with severe hepatic or renal impairment.
Special instructions
Special instructions
For patients with diabetes: 5 ml of solution contains 1.5 g of sorbitol, which corresponds to 0.12 bread units.
The drug contains alcohol in a concentration of 3%, which is 0.6 g per dose of the drug for adults (20 ml), respectively, for children (2.5 ml) – 0.075 g.
Impact on the ability to drive vehicles and operate machinery
Taking the recommended therapeutic doses (20 ml) does not affect the speed of the patient’s psychomotor reactions.
Significantly exceeding the recommended doses of a drug containing 3% alcohol can affect the speed of psychomotor reactions and pose a danger when driving a car or working with equipment.
Active ingredient
Active ingredient
Bromhexine
Composition
Composition
Active ingredient:
Bromhexine hydrochloride – 0.8 mg.
Excipients:
levomenthol,
hydrochloric acid 2 mol/l,
methyl parahydroxybenzoate,
ethanol 96%,
sorbitol,
purified water.
Contraindications
Contraindications
Hypersensitivity to the components of the drug;
a history of episodes of hemoptysis;
pregnancy and breastfeeding;
gastric ulcer (in the acute stage);
children under 3 years of age;
hereditary fructose intolerance (since the drug contains sorbitol)
Use with caution in patients with a history of gastric bleeding, bronchial disease accompanied by excessive accumulation of secretions, renal and/or liver failure.
The presence of alcohol in the drug (96%) may have an adverse effect on children, pregnant women, people with brain diseases, epilepsy, traumatic brain injury, and alcoholism.
If you have any of the diseases or conditions listed in this section, the patient should consult a physician before use.
Side Effects
Side Effects
Dyspepsia, including: nausea, vomiting; exacerbation of gastric ulcer and duodenal ulcer; allergic reactions; dizziness; headache; increased activity of “liver” transaminases in blood serum.
Interaction
Interaction
Bromhexine is not prescribed simultaneously with antitussives (including those containing codeine), as they may make it difficult to cough up sputum diluted by Bromhexine.
Bromhexine promotes the penetration of antibiotics (erythromycin, cephalexin, oxytetracycline, ampicillin, amoxicillin), sulfonamide drugs into bronchial secretions.
The drug is incompatible with alkaline solutions.
Overdose
Overdose
Symptoms: nausea, vomiting, diarrhea and other gastrointestinal disorders.
Treatment: there is no specific antidote. In case of overdose, it is necessary to induce vomiting, and then give the patient liquid (milk or water). Gastric lavage is recommended within 1 – 2 hours after taking the drug.
Storage conditions
Storage conditions
Store at a temperature not exceeding 25°C.
Keep out of the reach of children.
Shelf life
Shelf life
3 years.
Manufacturer
Manufacturer
Takeda GmbH, Germany
Additional information
| Shelf life | 3 years. |
|---|---|
| Conditions of storage | Store at a temperature not exceeding 25°C. Keep out of the reach of children. |
| Manufacturer | Takeda GmbH, Germany |
| Medication form | oral solution |
| Brand | Takeda GmbH |
Other forms…
Related products
Buy Bromhexin Nicomed, 0.8 mg/ml 150 ml with delivery to USA, UK, Europe and over 120 other countries.